Biology Basics
The purpose of this site is to explore biology and the things that make up biology. Feel free to suggest corrections or additions. You may follow updates to this page using this RSS feed and some RSS reader.
Big History
The universe is at least 13.7 billion years old and more than 100 billion light years in diameter.
We live on Earth which is part of a solar system with a star called the Sun. Earth and its star coalesced about 4.5 billion years ago. Earth’s solar system is part of the Milky Way galaxy, which is at least 12 billion years old, contains over 100 billion stars and 100 billion planets, and is over 100,000 light years in diameter (or about 0.0001% of the size of the universe).
There are over 100 billion galaxies in the universe.
Basics
This page assumes basic knowledge of math, algebra, geometry, and scientific notation.
Matter
The universe is made of matter which is something that has mass (m) and takes up a position in space.
Examples of matter are protons, neutrons, and electrons. Protons and neutrons each have a mass of about 1.6×10−27 kilograms (kg), whereas electrons have a much smaller mass of about 9.1×10−31 kg. Protons and neutrons each have a radius of about 0.8×10−15 meters (m), whereas electrons have a radius of less than 1.0×10−22 m.
Distance between two points is measured in meters using the International System of Units (SI; abbreviated from French), also known as the metric system. A prefix indicates the multiple of the unit. Common metric prefixes include exa- (E; 1018), peta- (P; 1015), tera- (T; 1012), giga- (G; 109), mega- (M; 106), kilo- (K; 103), milli- (m; 10-3), micro- (μ; 10-6), nano- (n; 10-9), pico- (p; 10-12), and femto- (f; 10-15). The radius of the proton and neutron could alternatively be described as 0.8fm. Common words include the micron which is 1 μm.
An object (or entity) is a collection of matter. An object has an inertia, meaning:
- An object at rest will stay at rest unless a force (F) is applied to push or pull it, and
- An object in motion will stay in motion unless a force is applied to push or pull it.
The speed of an object is a description of its movement and is the rate at which its position in space changes over a distance over a period of time (e.g. moving at 13 meters per second (s), or 13 m/s). A minute is 60 seconds. An hour is 60 minutes.
The velocity (v) of an object is a description of both its speed and its direction in space (e.g. moving at 13 m/s down).
The acceleration (a) of an object is the rate at which its velocity changes over a period of time (e.g. accelerating at 13 m/s over 1 second down, or 13 m/s2 down).
The momentum (p) of an object, or its “quantity of motion”, is its mass multiplied by its velocity (p=m×v).
If the mass of an object is constant, the force acting on it is the rate of change of its momentum over time (F=dp/dt); or, substituting momentum for acceleration and simplifying, its mass multiplied by its acceleration (F=m×a). The unit of force is the newton (N).
When objects touch each other, the perpendicular force their surfaces apply is called the normal force (Fn), and any parallel forces are called frictional forces (Ff).
When an object A exerts a force on another object B, then object B exerts an equal and opposite force on object A.
Electric Charge
Electric charge is the property of some forms of matter to create an electromagnetic field which applies a positive or negative force on other electrically charged matter, proportional to the distance between them. The magnitude of this force (named the electrostatic force) is calculated with Coulomb’s law (F=ke×((q1×q2)/r2)) which is Coulomb’s constant (ke) multiplied by the magnitudes of the two charges involved (q1 and q2) and divided by the square of the distance between the two objects (r2). This equation means that positive charges repel other positive charges, negative charges repel other negative charges, and positive charges attract negative charges (and vice versa). The unit of electric charge is the Coulomb (C).
Examples of electrically charged matter are protons, which are positively charged, and electrons, which are negatively charged. Protons and electrons have the same magnitude elementary electric charge, denoted 1e and -1e, respectively. Neutrons have no charge (electrically neutral).
Amperes (amps or A) are a measure of the flow of a certain number of elementary electric charges across a boundary in one second: 1 A = 1 C / 1 s.
An electromagnetic field creates electromagnetic radiation which is a wave of its force traveling (radiating) through space. A wave represents something that repeats over time at a frequency which is how often it repeats a cycle per unit time (e.g. one cycle per second). If the unit of time is one second, the unit of frequency is Hertz (Hz). Wavelength is the distance a wave covers over one cycle.
The force carrier for electromagnetic radiation is a photon. Photons are considered particles (which may also refer to objects) even though photons are massless. In a vacuum (space without matter), photons travel at the maximum speed of light (c), or approximately 3×108 m/s. Light is simply electromagnetic radiation; although, colloquially, light usually refers to visible light which is the subset of light that humans see (~390 to ~700nm wavelengths), where white is a combination of all colors and black is the lack of all colors.
Pigment is matter that absorbs certain wavelengths of light. Unabsorbed wavelengths are reflected giving the matter a color to observers. For example, a black pigment means all wavelengths were absorbed, a white pigment means none were absorbed, and a green pigment means all wavelengths but green were absorbed.
Fluorescent matter absorbs ultraviolet radiation (~10nm to ~400nm wavelengths) and emits visible light.
Refraction is the bending of a wave as it moves between areas of different densities (and thus different speeds). The refractive index is a ratio expressing the degree light is refracted from one density to another with a higher index meaning higher bending.
Energy
Energy (E) is the amount of work one object performs on, or transfers to, another object. Energy is often measured in Joules (J). One joule may be defined as J=N×m or a force of one Newton acting on an object in the direction of its motion for one meter.
Energy is either kinetic energy if an object is in motion (in most simple cases expressed as (m×v2)/2); or, potential energy, which may be thought of as stored energy.
Energy may neither be created nor destroyed, but only transformed. Mass may be converted to energy and vice versa through the mass-energy equivalence equation (E=m×c2).
Voltage
Electric potential is the amount of energy needed to move a unit of positive charge in an electromagnetic field to another point without producing acceleration.
Voltage (V) is the difference in electric potential between two points (i.e. the electric potential energy). Voltage is measured in Volts which are calculated using the potential energy divided by the charge, or V = (N×m)/C.
Pressure and Temperature
Pressure (P) is the normal force per unit area (A) applied to an object or P=Fn/A, often measured in Pascals (Pa) (in N/m2). An object under pressure has potential energy.
Atmospheric pressure is the pressure of a planet’s atmosphere (e.g. the pressure of air) on an object. The mass of air in the Earth’s atmosphere decreases exponentially with altitude so the atmospheric pressure decreases exponentially as an object rises above sea level.
Another unit of pressure is the atmosphere (atm) which is the pressure on Earth at sea level and it’s equivalent to 101,325 Pa.
Partial Pressure (p) is the pressure exerted by one gas within a mixture of gases. A gas diffuses from a region of higher partial pressure to lower partial pressure.
States of Matter
Volume is a quantity of three-dimensional space measured in m3. Density is mass per unit volume. Relative density is a ratio of densities. Specific gravity is a relative density to some reference substance.
In general, matter exists in one of four states and an object may change states in a phase change:
- Solid: Matter which has fixed volume and fixed shape, with its components close together and fixed in place. In equations, solid substances may be suffixed with (s).
- Liquid: Matter which has fixed volume and variable shape to fit its container, with its components close together but not fixed in place. In equations, liquid substances may be suffixed with (l).
- Gas: Matter which has variable volume and variable shape, both to fit its container, and its components are neither close together nor fixed in place. In equations, gas substances may be suffixed with (g).
- Plasma: Matter which has variable volume and variable shape, but also contains a large number substances with electric charge moving freely.
Heat
Heat (H) is a measure of the total quantity of kinetic energy. Temperature is a measure of the average kinetic energy of a set of objects. Absolute zero is the coldest state at which an object has minimal movement; however, practically, it may be assumed that all objects have some movement (or vibration) and thus some non-zero temperature.
Temperature or heat is either measured in degrees (°) of change on the scale of Celsius (°C) or Fahrenheit (°F), or in absolute terms on the scale of Kelvin (K).
Roughly, the scale of celsius is defined with 0°C being when pure water freezes at 1 atm, 100°C when pure water boils at 1 atm, and absolute zero is -273.15°C. The scale of fahrenheit is defined with 32°F being when pure water freezes at 1 atm, 212°F when pure water boils at 1 atm, and absolute zero is -459.67°F. Originally, the Kelvin scale was defined relative to Celsius, but is now defined as pressure invariant, with 0 as absolute zero, and 273.16K when pure water reaches its triple point. Kelvin was designed so that an increase of one Kelvin is equal to an increase of 1°C.
The lowest recorded surface temperature on Earth is 184K / -89.2°C / -128.6°F, the highest 331K / 58°C / 136.4°F, and the average 288K / 15°C / 59°F.
Heat capacity is the amount of energy that must be added or removed to achieve a uniform change in an object’s temperature, divided by the magnitude of the change.
Latent heat is the amount of energy an object absorbs or releases per unit volume during which the temperature of the object stays constant.
The latent heat of vaporization (or evaporation) is the amount of energy gained per unit volume below the boiling point to break intermolecular bonds and complete the phase change of liquid to gas. To do this, some molecules gain energy from their neighbors which is also called evaporative cooling.
The latent heat of melting is the amount of energy gained per unit volume at the freezing point to break intermolecular crystal bonds and complete the phase change of solid to liquid (freezing).
The latent heat of condensation is the amount of energy released per unit volume for a gas to complete the phase change to a liquid (condensation) as it cools.
The latent heat of freezing is the amount of energy released per unit volume at the freezing point to complete the phase change of liquid to solid.
Sublimation is the phase change from solid directly to gas.
Specific heat is the amount of heat that must be absorbed or lost for 1g of something to change its temperature by 1°C.
All matter above absolute zero temperature continuously emits some of its kinetic energy as photons of electromagnetic radiation called thermal radiation or heat. The temperature determines the emission spectrum of wavelengths of the electromagnetic radiation. Most matter may also absorb some of any incoming electromagnetic radiation.
If two objects touch with a path permeable to heat, then, all else being equal, the hotter object heats the cooler object through thermal conduction (or thermal convection depending on the state of matter) until they (or at least their touching surfaces) reach thermal equilibrium.
Thermodynamics
There are four laws of thermodynamics (starting with the zeroth law):
- If two systems A and B are in thermal equilibrium with a third system C, then A and B are in thermal equilibrium.
- Energy may be neither created nor destroyed, but only transformed.
- Energy transformation in a closed system increases entropy.
- Entropy approaches a constant value as the temperature of a closed system approaches 0 Kelvin.
Entropy is a measure of disorder in a system.
Atoms
Atoms are made of at least one proton and zero or more neutrons in the atom’s nucleus (both protons and neutrons may be called nucleons), and zero or more electrons orbiting the nucleus in electron shells. Electron shells are also called energy levels because the shell number represents the relative potential energy of electrons in that shell. As electrons are in shells farther from the nuclear proton(s), their potential energies increase because of the additional potential electrostatic force. Therefore, an electron moving to a shell closer to the nucleus must lose energy (e.g. heat) and an electron moving to a shell farther away must absorb energy (e.g. light). Shells are subdivided into subshells, and subshells are subdivided into orbitals (discussed later).
The number of nucleons is considered the atomic mass number (A) since an atom’s electrons’ masses are so much relatively lighter than the nucleons’ masses.
The number of protons is considered the atomic number (Z) and categorizes the atom in a class called a chemical element (e.g. Carbon is the chemical element class for any atom which has 6 protons; an atom is an instance of the chemical element). The atomic number of a chemical element is sometimes placed in a subscript to the left of the element symbol; for example, 6C, although this is redundant because the symbol C implies Z. Chemical elements and their reactions are the basis of chemistry.
The number of neutrons defines the element’s isotope which is represented as the element name followed by its atomic mass number (e.g. Carbon-14), so the number of neutrons may be deduced from the isotope name by subtracting the number of protons. An isotope may be abbreviated with the atomic mass number in a superscript to the left of the element symbol; for example, 14C for Carbon-14.
Atomic mass (mA) is measured in unified atomic mass units (u or amu), also known as daltons (Da) and 1u is approximately the mass of a nucleon. For various reasons, 1u is defined more strictly as 1/12th of the mass of a Carbon-12 atom. Relative atomic mass (Ar) is the weighted average mass of a set of atoms. Standard atomic weight (Ar,std) is Ar on Earth, reflecting the weighted average of isotope masses of an element on Earth.
If an atom has an equal number of protons and electrons, then it is electrically neutral. If an atom has an unequal number of protons and electrons, then it is ionized. If an atom has more protons than electrons, then it’s positively electrically charged, called a cation, and symbolized as E+ (where E is the element symbol, discussed below). If an atom has more electrons than protons, then it’s negatively electrically charged, called an anion, and symbolized as E-. If the ion is more than one electron away from the neutral element, then the + or - is preceded by that number, e.g. E2+.
Chemistry
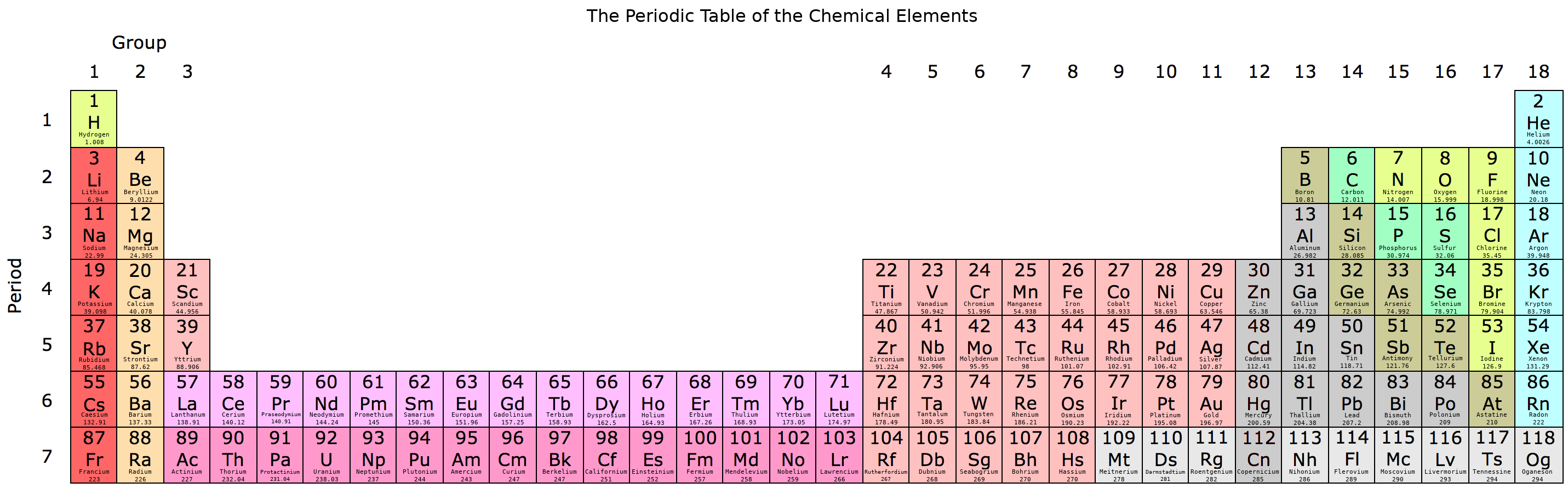
The Periodic Table of Elements
There are 118 known chemical elements, 92 of which have been observed naturally, the rest synthesized, and only four elements (Carbon, Oxygen, Hydrogen, and Nitrogen) make up about 96% of a human’s mass, with another 21 required in small amounts (mostly Calcium, Phosphorus, Potassium, Sulfur, Sodium, Chlorine, and Magnesium).
The periodic table is a way to organize and understand the chemical elements based on observed patterns. The elements are ordered by atomic number Z from left to right, and starting again at the left when going down.
Within each square, the atomic number is at the top, followed by the element’s symbol, followed by the element name, followed by its standard atomic weight (Ar,std):
The main reason for describing elements in such a way has to do with electron configuration patterns and the behaviors they may cause (described later).
Rows are called periods and describe a new electron shell which accumulates on top of any previous periods’ shells. This outermost shell is known as the valence shell which generally contains the valence electrons that may be reactive (some ionized elements complicate this picture since they contain one less electron and effectively drop down a shell - for example, the Lithium cation - but the table is a conceptual starting point from neutral atoms).
Columns are called groups and generally group by the number of valence electrons, and thus generally group by similar behavior.
Each electron shell in an atom has a maximum number of electrons (2×ShellNumber2) before the next shell starts. Each shell has a distinct energy level and is broken down into subshells which have a maximum number of electrons before the next subshell starts. Each subshell is broken down into orbitals of up to 2 electrons each. Additional electrons fill empty orbitals in a sub-shell before pairing up with an electron in an existing orbital. A full orbital is called an electron pair. A lone pair of electrons is a filled valence orbital of two electrons that are not shared with another atom (sometimes noted with a :). Electron configurations are represented by the accumulation of subshells up to the total number of electrons, with each subshell described by the shell number, followed by the subshell name, followed by the number of electrons in that subshell in a superscript. The subshell names are:
- s: For groups 1 and 2 (or group 18 for Helium) only, at most 2 electrons.
- p: Starting at period 2, for groups 13-18 only, at most 6 electrons.
- d: Starting at period 4, for groups 3-12 only, at most 10 electrons.
- f: Starting at period 6, in between groups 3 and 4 only, at most 14 electrons.
- […]
Examples of electron configurations for the first 11 neutral elements:
Hydrogen (1): 1s1 Helium (2): 1s2 Lithium (3): 1s2 2s1 Beryllium (4): 1s2 2s2 Boron (5): 1s2 2s2 2p1 Carbon (6): 1s2 2s2 2p2 Nitrogen (7): 1s2 2s2 2p3 Oxygen (8): 1s2 2s2 2p4 Florine (9): 1s2 2s2 2p5 Neon (10): 1s2 2s2 2p6 Sodium (11): 1s2 2s2 2p6 3s1 [...]
Instead of writing the full details of long electron configurations, a common practice is to start with the previous group 18 element in [brackets] followed by the rest of the element’s electron configuration. For example, Sodium (element #11) may be written as [Ne] 3s1.
When d and f orbitals are filled, they backfill the previous shell. For example, Scandium’s (element #21) electron configuration is [Ar] 4s2 3d1.
The electrons that tend to cause an atom to chemically react are those electrons with the highest energies (the farthest distances from the nucleus) and are usually those in the valence shell. The exceptions are elements with d or f orbitals because even though those backfill the previous, non-valence shell, they may have higher energies than the s orbital electrons of the valence shell; however, these energies decrease moving right on a period, so the number of these valence electrons is limited.
Generally, atoms tend to be chemically reactive when their valence electrons do not complete their valence shell and thus the atom is unstable (high energy). All elements above period 1 generally want 8 electrons in their valance shell and this heuristic is called the octet rule. Atoms tend to gain, shed, or share pairs of electrons as needed to reach a full and stable (low energy) set of valence electrons. This is one of the most important aspects of chemistry and means that groups have generally similar behaviors since they’re generally grouped by the number of valence electrons. There are 18 numbered and 6 named groups for convenience:
- Group #1: Alkali metals - Highly reactive because they want to lose an electron to drop to the previous period’s full set of valence electrons.
- Group #2: Alkaline earth metals - Somewhat reactive because they want to lose two electrons to drop to the previous period’s full set of valence electrons.
- Group #15: Pnictogens
- Group #16: Chalcogens - Somewhat reactive because they want to gain two electrons or share pairs of electrons to fill their set of valance electrons.
- Group #17: Halogens - Highly reactive because they want to gain an electron or share a pair of electrons to fill their set of of valence electrons.
- Group #18: Noble gases - Generally not chemically reactive (inert) because the set of valence electrons is full.
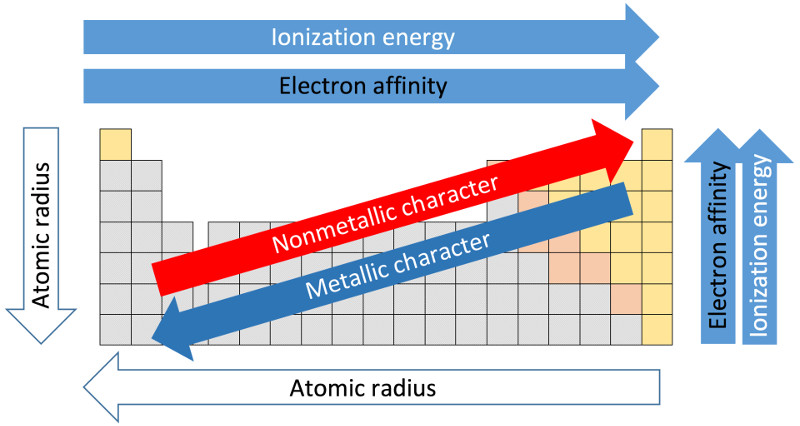
The radius of an atom increases from top to bottom as electron shells are added; however, moving left to right, the radius of an atom decreases as the additional protons draw in the additional electrons.
Ionization energy (or cationization energy) is the amount of energy needed to remove an electron from an element (and form a cation). Energy is required to remove the electron because the electron is attracted to its element’s proton(s). Following from the octet rule, ionization energy is lowest on the left of the table because those elements want to lose electron(s) to achieve a full set of valence electrons, and ionization energy generally increases from left to right as additional protons add more pull to the electrons. Ionization energy decreases from top to bottom because valence electrons are farther from the protons (to which they’re attracted) and thus the electrons are easier to peel off.
Electron affinity (or anionization energy) is either:
- The amount of energy released when an electron is added to an incomplete set of valence electrons. Energy is released because any time an electron drops into a new orbital, it causes the release of electromagnetic radiation energy in the form of a photon. Or,
- The amount of energy spent adding an electron to create a new subshell or adding an electron to a subshell which only has a single electron in each of its orbital pairs (e.g. Nitrogen). Energy is needed to overcome the last subshell’s stability.
Generally, electron affinity increases from left to right (except for those with stable last subshells) because right-most elements want additional electrons to achieve stability.
Electronegativity is the tendency of an atom to attract electrons to its valence shell (closely related to electron affinity). It follows from the octet rule that atoms increase in electronegativity from left to right. Electronegativity decreases from top to bottom because the valence shell is farther away from the positively charged nucleus and thus there’s less pull to bring in additional electrons. Electropositivity is the opposite of electronegativity.
Most elements are metals (although there are only two named metal groups): they are toward the left side of the periodic table (with the exception of Hydrogen), have low ionization energies, low electron affinity, are highly electrically conductive, ductile, and generally solid at standard temperature.
Nonmetals are the opposite of metals: they are toward the right side of the table, have high ionization energies, high electron affinities, and are not very electrically conductive.
There are a handful of Metalloids which have properties of both metals and nonmetals and run down a diagonal in the p-block (e.g. Boron, Silicon, etc.).
A transition metal is any element with a partially filled d sub-shell (groups 3-11).
In summary, although with various exceptions, the broad trends of atomic size, ionization energy, electron affinity and metallic character may be visualized as:
Big History (Continued)
After the Big Bang, the universe was mostly made of hydrogen, helium, and lithium (the first three elements), some of which combined into plasma stars held together by gravity. At high enough temperatures inside stars, hydrogen atoms undergo nuclear fusion where nuclei (and their protons and neutrons) combine to produce helium. As the hydrogen is used up to create helium, the star’s temperature rises and allows for the fusion of helium into carbon and oxygen and some neon and heavier elements. As the star temperature continues to increase, nuclear fusion produces elements up to iron. The remaining elements in the universe were produced by certain stars’ s-processes and stars’ explosions in a supernova or collapses into a black hole or neutron star, although elements may be transformed from one element into another under certain conditions.
Chemistry (Continued)
Two or more atoms may be held together by chemical bond(s). Some types of bonds are:
- A covalent bond defines a molecule and occurs when atoms share one or more pairs of electrons in their valence shells. For example, Hydrogen wants to gain an electron and Oxygen wants to gain two electrons, so it’s common for two Hydrogens to each share their electron with one of the valence electrons in the Oxygen to form an Oxygen bonded with two Hydrogens, or water. A molecule is considered a chemical compound if it’s made of more than one type of element. The strongest form of a covalent bond is a sigma bond (σ bond). Another, weaker form of a covalent bond is a pi bond (π bond).
- A coordinate covalent bond (or dative bond) defines a covalent bond in which one atom donates both electrons to the bond. Such bonds form a coordination complex. An example is a metal cation which has a coordinate covalent bond to an atom with a lone pair (also called a ligand).
- An ionic bond defines an ionic compound or salt when one atom transfers electron(s) to another, creating a cation and anion, which then causes electrostatic attraction of the oppositely charged ions. For example, the alkali metal Sodium (Na) wants to lose an electron, and the halogen Chlorine (Cl) wants to gain an electron, so Na may give its electron to Cl, thus making Na+ and Cl- and then those two ions may bond due to the electrostatic force, forming NaCl (otherwise known as table salt).
- A metallic bond amongst positively charged metal cations in a sea of shared electrons.
- Chelation bonds ions or molecules to metal icons.
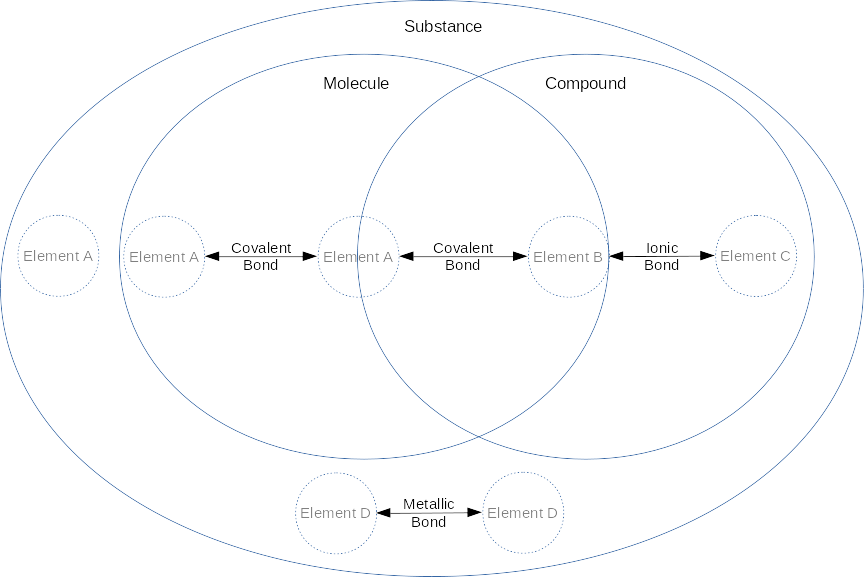
A chemical substance is a set of one or more elements, molecules or compounds of the same composition (i.e. “pure”). A substance cannot be separated through physical means other than breaking chemical bonds (interpret the venn diagram by the location of the bond arrows):
A mixture is a combination of different substances. A mixture is homogenous if its substances have the same proportions throughout (e.g. air), or otherwise heterogeneous.
A molecular entity is a single instance of a part or whole of a molecule, such as an atom, ion, or molecule. A set of identical molecular entities (in other words, a class of molecular entities) is a chemical species. For example, a single water molecule is a molecular entity, but all the instances of a water molecule in some context is a chemical species. Relatedly, an elementary entity is similar to a molecular entity but may also be an electron, particle, or group of particles.
A free radical is a substance with at least one unpaired valence electron (e.g. a halogen), making it highly chemically reactive.
A substance may be described in many ways, all of which may represent either an entity or a species:
- A molecular formula describes the number of atoms of each element in each molecule in a subscript to the right of the element symbol (or 1 if the subscript is omitted). For example, the molecule H2O represents two Hydrogen atoms bonded with one Oxygen atom.
- An empirical formula is the molecular formula with the ratios of elements reduced to the simplest form. For example, a molecule of Benzene has a molecular formula of C6H6, but the empirical formula is CH.
- A trivial or retained name; for example, Water represents H2O (in the case of water, the molecular and empirical formulas are the same).
- Various structural formulas that describe the two-dimensional (2D) or three-dimensional (3D) structure of the molecule. For example, a covalent bond is represented with a long dash (–) such as
H–O–Hfor H2O. As another example, a double covalent bond is represented with a double dash (=) such asO=C=Ofor CO2.- Wedge-hash diagrams (or wedge-dash diagrams or Natta projections): The filled wedge is projected toward the viewer (in front of the page). The dashed wedge is projected away from the viewer (behind the page).
- Fischer projections
- Unspecified stereochemistry: Wavy single bonds are unknown or unspecified stereochemistry. Stereochemistry is the study of substances with the same formula but with different positions of atoms in space that cannot be rotated around a single bond to match each other (discussed in detail later).
- Skeletal formulas
- An ionic compound name with a set of element names with any cation first. The anion in such a salt takes the suffix -ide. For example, NaCl is Sodium Chloride.
- If the cation is a transition metal, then it may be followed by roman numerals in parentheses (type-I compounds, type-II compounds or type-III compounds) which represents the net positive charge of that cation; for example, Iron(III) Oxide is Fe2O3 because the III means that Iron is Fe3+ and gave 3 extra electrons, and since each Oxygen atom needs two electrons, there should be two Iron atoms, making 6 extra electrons, which means there are three Oxygen atoms, each taking 2 of those 6 extra electrons.
- If a molecule is ionic and contains Oxygen, suffixes may be used to differeniate between the same compound but with a different number of Oxygens: The -ate suffix is used on the most common molecule, the -ite suffix is used with one less Oxygen than the -ate molecule, the hypo- prefix along with the -ite suffix is used with two less Oxygens, and the per- prefix is used with one more Oxygen. For example, Chlorate is ClO3-, Chlorite is ClO2-, Hypochlorite is ClO-, and Perchlorate is ClO4-.
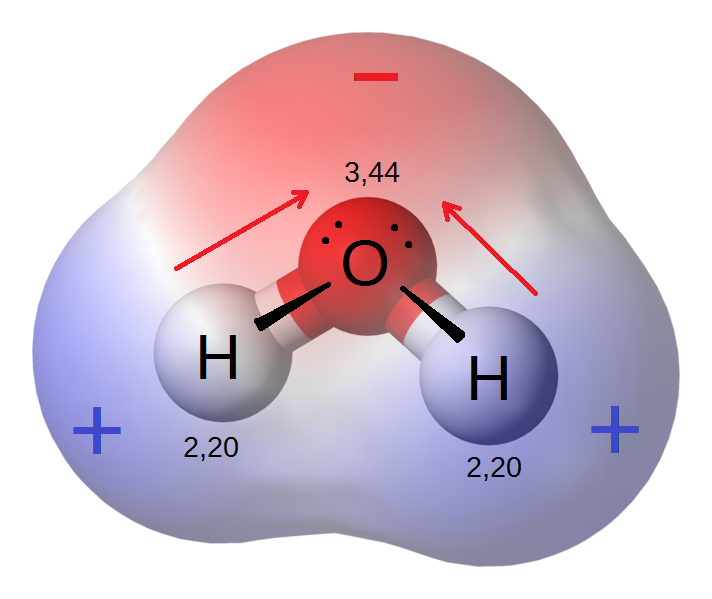
When substances of different electronegativities are bonded, electrons will tend to be drawn closer to the atoms with higher electronegativities, thus leading to positive or negative partial charges (δ+ or δ-, respectively), creating a polarized (or polar) bond. For example, in the water molecule, Oxygen is more electronegative than the Hydrogens, so there’s a partial negative charge at the end of the Oxygen away from the two Hydrogens, and partial positive charges on the opposite ends of the Hydrogens:
Oxidation states are a conceptualizatized simplification of covalent bonds as ionic bonds. Oxidation states assume full hogging of electrons in a polar molecule. For example, in water, Oxygen would have an oxidation state of -2 since it hogs both Hydrogens’ electrons, and each Hydrogen would have an oxidation state of +1.
Reduction is the process of gaining electrons. Oxidation is the process of losing electrons. The atom losing the electrons is said to be oxidized (even if by something other than Oxygen; the term originates from Oxygen’s high electronegativity), i.e. losing electrons, and the atoms doing the oxidation are said to be reduced by the other elements, i.e. reducing the electrons of the oxidized atoms. This is called a redox (reduction and oxidation) reaction because both occur. Oxidation frequently involves gaining an Oxygen or losing a Hydrogen. Reduction frequently involves losing an Oxygen or gaining a Hydrogen. A simple example of a redox reaction is Na and Cl creating Na+ and Cl-. An electron loses potential energy as part of reduction because it moves towards a more electronegative atom, and this energy is released and can be harvested by surroundings.
Intermolecular forces describe attraction and repulsion forces between substances and are relatively weaker than intramolecular forces:
- Dipole-dipole forces occur when the opposite partial charges of the dipoles of two polar molecules (or different parts of a large molecule) attract each other. For example, two water molecules have a dipole-dipole interaction between the partial negative end of the Oxygen of one molecule and the partial positives of the Hydrogens of the other molecule. When this interaction occurs with Hydrogren, this may be called Hydrogen bonding since it’s the strongest form of dipole-dipole interaction.
- Van der Waals forces
- London dispersion forces occur transiently at short distances as electrons happen to be in parts of their orbitals which create partial charges and create temporary dipole-dipole interactions.
Intercalation is the reversible inclusion of a substance into layered material.
A [dye][] is matter that chemically bonds to a substrate and, like a pigment, abosrbs certain wavelengths of light.
Moles
A mole (mol) is defined as the number of elementary entities in a substance as there are atoms in 12g of 12C. Moles are essentially a way to convert between amus and grams. There are ~6.022140857×1023 (Avogadro constant) atoms in 12g of 12C. Therefore, 1 mol 12C = 12g.
Since 1u is 1/12th the mass of one 12C atom, then 1 mol 1u = 1g; therefore, 1 mol of isotope mAE ~= mA grams. If a substance doesn’t refer to a particular isotope, then standard atomic weight (Ar,std) is generally used instead of mA. Molecular mass is simply the sum of atomic masses of a molecule.
For example, Ar,std of H is 1.008, and Ar,std of O is 15.999, so 1 mol H2O ~= (1.008×2)g + 15.999g ~= 18.015g. The molar mass (M) of a substance is simply this relationship in terms of g/mol, so M(H2O) ~= 18.015 g/mol.
Chemical Reactions
A chemical reaction occurs any time a chemical bond is created or broken. A chemical equation describes a reaction with reagents (or reactants) on the left-hand side of the equation which yields (→) the product(s) of the chemical reaction on the right-hand side of the equation (an adduct is a type of product where there are no products other than the adduct product). For example, the chemical equation for the reaction of molecular hydrogen and molecular oxygen plus input energy (E) yields water and output energy E’:
2H2 + O2 + E → 2H2O + E'
Enthalpy is the heat transferred (ΔH) during a constant pressure process. An endothermic reaction occurs when the system absorbs heat energy (positive ΔH) from its surroundings. An exothermic reaction occurs when the system releases energy (negative ΔH).
A reagent which is attracted to electrons is called an electrophile (i.e. a lewis acid). A nucleophile is a reagent which donates electrons (i.e. a lewis base) to an electrophile.
A chemical equation must be balanced using stoichiometry because the total number of atoms of each element must be the same on both sides. A stoichiometric coefficient to the left of a substance represents the number of substances or moles of that substance in the chemical reaction.
Chemical reactions may also be reversible and go in both directions, signified by a double arrow (⇌), always tending towards reaching dynamic chemical equilibrium where the rates of reactions in both directions are equal. For example:
HCO3- + H+ ⇌ H2CO3
In a reversible chemical reaction such as aA + bB ⇌ cC + dD, the equilibrium constant (keq) measures the extent to which reagents are converted to products and equals ([C]c × [D]d) / ([A]a × [B]b), ignoring the solvent and any solids, where the a, b, c, and d coefficients are the mole ratios. Given that molarity depends on temperature, keq is a function of temperature. This constant describes the relative proportions of concentrations of reagents and products at equilibrium.
Gibbs free energy (G) measures the work that a system can do (ΔG = ΔH - T×ΔS where ΔS is the change in entropy). If the change in G (ΔG) for a chemical reaction at a temperature T is known, Keq can be calculated as ΔG = -R×T×ln(Keq).
Reactions that don’t require input energy are called spontaneous or exergonic (negative ΔG, meaning that the products store less energy than the reagents and thus the reaction favors the products spontaneously without input energy), although they don’t necessarily occur rapidly.
Reactions that require input energy are endergonic (positive ΔG):
Endergonic Reaction
⎪
E ⎪
n ⎪ ____
e ⎪ / \________
r ⎪ / products
g ⎪________/
y ⎪reagents
⎪________________________
Time
A coupled reaction is when the energy released by an exergonic reaction drives an endergonic reaction.
Given a balanced chemical equation and the relationship of moles to atomic weights, and a mass of one substance, composition stoichiometry may be used to determine the mass of other substances in the equation. For example, in the following equation:
Fe2O3 + 2Al → Al2O3 + 2Fe
If we know there are 85g of Fe2O3, since each of those molecules is about 160u, Fe2O3 is about 160g/mol, so first convert 85g to moles using dimensional analysis by multiplying by 1mol/160g to cancel the g, and therefore, 85g divided by 160g/mol is 0.53 mols. Since there are two moles of Al for every 1 mole of Fe2O3, 2×0.53 = 1.06 mols of Al would be needed to react with all of the Fe2O3. Given Al’s atomic weight is about 27u, 1 mole is about 27g, so 1.06×27 = 28.62g of Al would be needed to react with all of the Fe2O3.
Le Chatelier’s principle (or the equilibrium law) states that the reaction tends towards equilibrium even after any changes to concentration, temperature, volume, or pressure (thus why it’s called dynamic equilibrium):
- If the temperature increases in an endothermic reaction, the system will favor the forward reaction.
- If the temperature decreases in an exothermic reation, the forward reaction will be favored to match the previous temperature.
- If the concentration of a substance increases, the reaction will favor the other side, decreasing the concentration of the subtance, and increasing the concentration(s) of the other side.
- If the concentration of a substance decreases, the reaction will favor the side of the decrease, increasing the concentration of the substance, and decreasing the concentration(s) of the other side.
- If volume decreases, concentrations will increase, so the reaction will favor the side that generates more total moles (i.e. creating more of the side that’s more likely to react).
- If volume increases, concentrations will decrease, so the reaction will favor the side that has fewer total moles (i.e. creating more of the side that’s less likely to react).
Water
Water is a particularly imporant molecule. Since it’s a polar molecule, it has a tendency to create Hydrogen bonds with nearby water molecules (or other ions or dipoles). This phenomenon is called cohesion. Water also has a greater surface tension (how difficult it is to stretch or break the surface of a liquid).
Water has a high freezing point, high boiling point, high heat capacity, high specific heat, and high latent heat because of the additional energy needed to overcome its Hydrogen bonding. Water’s latent heat of vaporization is much greater than its latent heat of melting because vaporization needs to break most or all of the Hydrogen bonds whereas melting just needs to break the general crystal structure. Cold water holds gases better than warmer water. Frozen water is less dense than liquid water because of additional Hydrogen bonding increasing the volume of the same number of water molecules relative to liquid.
Freezing-point depression is the process of reducing the freezing point by adding a solute (such as salt) because more energy and/or time is required for molecules to Hydrogen bond to form a crystalline solid and freeze despite the solutes.
A calorie (cal) is the amount of heat energy it takes to raise the temperature of 1g of water by 1°C (conversely, the amount of heat energy released when 1g of water is cooled by 1°C). 1 calorie also equals ~4.184 J (conversely, 1 J ~= 0.239 cal). Therefore, the specific heat of water is 1 cal per 1g per °C.
Substances that are ionic or polar are hydrophilic meaning that they have a high affinity for water. Substances that are non-ionic and non-polar are hydrophobic meaning that they have a low affinity for water. Substances that are amphipathic have both hydrophobic and hydrophilic regions.
Frozen water is less dense than liquid water because at higher temperatures, the higher average kinetic energy means water molecules may more freely disassociate Hydrogen bonds between molecules whereas at lower temperatures, the Hydrogen bonding between water molecules cannot be as easily disassociated and this creates a more spaced-out lattice-type structure which is less dense.
Dessication occurs when something is extremely dry.
Solutions
A solution is a homogenous mixture with solute(s) dissolved into a solvent (the largest proportion substance). A solvent is often a liquid and if such a liquid is water, the solution is referred to as an aqueous solution (in equations, sometimes denoted with (aq)). A suspension is a heterogenous mixture where one substance eventually settles, whereas colloids are heterogenous mixtures that don’t settle (emulsions are colloids of liquids). As temperature of a solution decreases and pressure increases, the solubility of gases increases (Henry’s Law).
A saturated solution is a solution with a solute that dissolves until it is unable to dissolve anymore, leaving the undissolved substances at the bottom. An unsaturated solution is a solution with less solute than a saturated solution which completely dissolves, leaving no remaining substances at the bottom. A supersaturated solution is a solution with more undissolved solute than a saturated solution because of its tendency to crystallize and precipitate.
Precipitation is the process of converting a substance from a solution into a solid. The precipitate is the solid and the remaining solution is the supernate (or supernatant).
A litre (L) is a unit of volume equal to 1m3/1000. 1 litre of water is approximately 1 kg under standard conditions (e.g. 25°C).
The amount of a substance is usually measured in mass, moles or volume (e.g. 1g of salt in water). The concentration of substance A mixed into substance B is the amount of substance A divided by the total volume of substance B. Molarity (or molar concentration) (M) is the number of moles of a solute per liter of solution, abbreviated with square brackets around the solute (e.g. [Cl-]). Molality is the number of moles of solute per kg of solvent.
A hypertonic solution has a higher concentration of a solute compared to another solution. A hypotonic solution has a lower concentration of a solute compared to another solution. An isotonic solution has the same concentration of a solute compared to another solution. Like a dynamic equilibrium in chemical reactions, even in an isotonic solution, solvent and solution may move back and forth, but the rates of movement are equalized.
In solution, molecules naturally diffuse from areas of higher concentration to areas of lower concentration if possible. This is called moving down or with the concentration gradient. Osmosis is a particular type of diffusion which creates an osmotic gradient of a solvent from a hypotonic area to a hypertonic area because the concentration of the solvent is lower in the hypertonic area. Osmotic Pressure is the minimum pressure which needs to be applied to a solution to prevent the inflow of solvent across a membrane. An osmole is the number of moles of a solute that contribute to the osmotic pressure of a solution that contribute to osmotic pressure. Osmolarity is the number of osmoles of solute per liter of solution, with the difference from molarity being that some substances dissolve in solution and don’t affect osmotic pressure.
Water Potential (Ψ; in megapascals) predicts which direction water will move, taking into account solute concentration (Ψs) and physical pressure (Ψp).
The ratio of a solute to a solution is sometimes described in a parts-per notation such as parts-per thousand (‰, or permille, or 10-3, but not ppt which is parts-per trillion), parts-per million (ppm or 10-6), parts-per billion (ppb or 10-9), parts-per trillion (ppt or 10-12), or parts-per quadrillion (ppq or 10-15).
Acids and Bases
Protium is the most common isotope of Hydrogen on Earth with one proton and zero neutrons (1H). Deuterium is an isotope of Hydrogen with one proton and one neutron (2H). A hydron is a cationic Hydrogen with 0 electrons (1 or 2H+). A hydride is an anionic Hydrogen. A protium hydron (1H+) is often just called a proton since it’s just a proton with no neutrons nor electrons.
Hydroxide is the anionic molecule OH-. Hydronium is the cationic molecule H3O+.
There are multiple ways to define acids and bases:
- A Brønsted-Lowry acid is a substance capable of donating a proton. A Brønsted-Lowry base is a substance with a lone pair of electrons capable of accepting a proton (or indirectly, a substance that dissociates into hydroxide ions which then accepts the protons). A conjugate acid is the product that accepted protons (because it can then give that proton back in the reverse direction), and a conjugate base is the product that donated protons. For example, water is amphoteric meaning it can be either an acid or a base.
- A Lewis acid is a substance that has an empty electron orbital and may accept an electron pair. For example,
H+has an empty electron orbital. A Lewis base is a substance that has a lone pair of electrons not involved in bonding that it may donate. - An Arrhenius acid is a substance that tends to react in an aqueous solution to increase the concentration of protons (which tend to create hydronium). For example,
HCl(aq) + H2O(l) ⇌ Cl-(aq) + H3O+(aq). An Arrhenius base is a substance that tends to react in an aqueous solution to increase the concentration of hydroxide. For example,NaOH(aq) + H2O(l) → Na+(aq) + H3O+(aq).
The Arrhenius definition is limited to aqueous solutions whereas the Brønsted-Lowry and Lewis definitions are more general.
A substance that is basic is also sometimes referred to as alkaline or an alkali.
A strong acid or base dissociates completely in aqueous solution (→), whereas a weak acid or base dissociates partially (⇌).
A substance that is acidic in a neutral solution will have an overall negative charge. A substance that is basic in a neutral solution will have an overall positive charge.
A small proportion of water molecules in liquid water will tend to autoionize and form hydronium and hydroxide ions (H2O + H2O ⇌ H3O+ + OH−). For pure water, at the equilibrium point under standard conditions (i.e. 25°C), [H+] = [H3O-] = 10-7 M.
The pH scale is a way to describe how acidic or basic a mixture is without using exponents, and it’s just the cologarithm (or negative logarithm) of molarity: pX = -log [X]. pH is the cologarithm of H+ and describes how acidic the mixture is. Conversely, pOH is the cologarithm of OH- and describes how basic the mixture is. (Note: Do not confuse this pX notation with the pX notation [with italicized p] which describes partial pressure; e.g., pCO2). When pH = pOH (in other words, [H+] = [OH-]), the mixture is described as neutral. Mixtures with [H+] > [OH-] are acidic. Mixtures with [H+] < [OH-] are basic. In any mixture, [H+]×[OH-] = 10-14 (in other words, pH + pOH = 14). As the molarity of protons increases (e.g. stomach acid is between ~10-1.5 to ~10-3.5), the pH value decreases and thus acidity increases. Therefore, mixtures with a pH below 7 are acidic and basic above 7.
A buffer is a substance that minimizes changes to pH by accepting protons when in excess and donating protons when in deficit. Generally, a buffer is a molecule with both an acid and a base, and its equilibrium constant controls its behavior in buffering the mixture. For example, carbonic acid yields a bicarbonate ion and a proton: H2CO3 ⇌ HCO3- + H+.
Three-Dimensional Structure
The size and structure of a substance is key to the way it functions because of the implied likelihoods of chemical reactions with other substances. A commonly used phrase is that structure implies function.
Although substance structures are often described by molecular, empirical, or two-dimensional formulas for simplicity, actual substance structures are three-dimensional.
Isomers are substances that have the same molecular formula but different structures.
Structural isomers differ in their covalent arrangements. For example, butane (C4H8) is:
H H H H | | | | H–C–C–C–C–H | | | | H H H H
Whereas isobutane (also C4H8) is:
H–C–H H ⎪ H | ⎪ | H–C–C–C–H | | | H H H
Geometric isomers (or cis-trans isomers) have the same covalent arrangements but differ in spatial arrangements. For example, the following two molecules are geometric isomers of each other. If the Xs are on the same side, it’s a cis isomer, and if they’re on opposite sides, a trans isomer:
H H \ / C=C / \ X X H X \ / C=C / \ X H
A chiral substance may have multiple isomers, each of which is called an enantiomer which are geometric isomers which cannot be superimposed on each other and this can have functional implications. The two forms are designated L and D isomers (L-form and D-form) from the Latin for left and right (levo and dextro). R and S configurations can describe substances with multiple chiral carbons. An asymmetric carbon (or chiral carbon) is a carbon attached to four different types of atoms or groups.
Conformations are isomers in which the difference between molecules is rotation around a single bond and does not depend on different bonding.
Common Molecules
Ammonia is NH3. It has three covalent bonds with Hydrogen which leaves one lone electron pair (because there are 5 total valence electrons: 2s2 + 2s3). This lone electron pair makes it a base because it can accept protons. Ammonia is also polar because Nitrogen is much more electronegative than Hydrogen, but also because the shape of Ammonia is pyramidal. If it was planar, the dipoles would cancel out, but the lone pair pushes the other Hydrogens out into a pyramidal structure.
A tetrahydride is a group 14 element (e.g. C, Si, Ge, Sn, Pb, etc.) which is saturated with hydrogens (e.g. CH4). Tetrahydrides are considered non-polar.
Organic Chemistry
An organic compound is an ambiguous term that describes a compound that contains Carbon and is related to organisms. An organism is an object with the properties of life. The definition of life is controversial. The physicist Erwin Schrödinger proposed that the key element of life is the fight against entropy.
The original definition of organic was loose because Carbon Dioxide (CO2) is considered inorganic even though it contains Carbon and is produced by some organisms. In addition, organic compounds may be synthesized outside of organisms. The modern definition of organic retains these ambiguities, but in general, most Carbon-based compounds are considered organic (with exceptions such as CO2 sustained for historical reasons). Carbon has four unpaired valence electrons and can create bonds in four different, equally spaced directions, allowing for a wide variety of compounds. Other elements can form 4 or more bonds but they are larger which limits their permutations.
Hydrocarbons are organic molecules composed of Carbon and Hydrogen atoms. The electronegativities between Hydrogen and Carbon are close enough (2.2 and 2.55, respectively, on the Pauling scale) that hydrocarbons are generally considered non-polar and therefore generally hydrophobic.
Hydrocarbons are either aromatic (also known as arenes) or aliphatic (also known as non-aromatic). Aromatics have cyclic (in alternating single and double covalent bonds), planar arrangements (such as Benzene) and thus are very stable and unreactive, whereas most aliphatics are acyclic and less stable. Only some aromatic substances have pleasant smells.
A saturated hydrocarbon has no double or triple bonds (i.e. it’s saturated with the maximum number of Hydrogens). An unsaturated hydrocarbon has double or triple bonds between Carbons.
It is common to omit Carbons and Hydrogens in structural formulas for simplicity. For example, the structural formula for Cyclohexane (C6H12) is a ring:
H H \ / H C H \ / \ / C C /⎪ ⎪\ H ⎪ ⎪ H H ⎪ ⎪ H \⎪ ⎪/ C C / \ / \ H C H / \ H H
This can be simplified into a simple hexagon where each vertex is an implied Carbon atom with two Hydrogens:
⬡
Similarly for structures with double covalent bonds, the ring can be simplified with double lines. For example, the structural formula for Benzene (C6H6) is a ring:
H
|
C
/ ⑊
H–C C–H
║ ⎪
H–C C–H
\ ⫽
C
|
H
This can be simplified into the following hexagon with three double bonds where each vertex is an implied Carbon atom with one Hydrogen:
⌬
Naming Organic Compounds
Greek letters are sometimes used in numbered names (in the same way that some numbered lists use a, b, c, ...). The first 10 Greek letters are (lower case followed by upper case):
- Alpha: α Α
- Beta: β Β
- Gamma: γ Γ
- Delta: δ Δ
- Epsilon: ε Ε
- Zeta: ζ Ζ
- Eta: η Η
- Theta: θ Θ
- Iota: ι Ι
- Kappa: κ Κ
The longest linear covalently bonded chain (carbon skeleton) of Hydrocarbons may be called a backbone chain (or main chain) from which the rest of the molecule builds off of. Any other smaller branches (in place of Hydrogens for the branch points) are called side chains, substituents, or pendant chains.
Any part of a molecule may be omitted and represented with the letter R (or R’, R’’, etc. if there are multiple substitutable parts), meaning remainder.
A moiety is a part of a molecule which is a common pattern of atoms that may be found in many other molecules.
The -yl suffix is used for free radicals or moieties (with an unpaired valence electron) that can be side chains substituting for a single Hydrogen on a backbone. The -ylidene suffix is the same but when substituting two Hydrogens on the backbone for a double bond. The -ylidyne is when substituting three Hydrogens on the backbone for a triple bond.
An alkane is an acyclic, unbranched saturated hydrocarbon. An example is Methane which is CH4 and is the main component of natural gas. A straight-chain (non-branched) alkane has the suffix -ane.
An alkene (or olefin) is an unsaturated alkane with at least one C=C double bond. An alkyne is an unsaturated alkane with at least one C≡C triple bond. The position of the double or triple bond is written at the start of the name or before the -ene or -yne suffix. If there are more than one such bonds, the positions are comma separated (e.g. 2,4-pentadiene). A substance that’s both an alkene and alkyne has the suffix -enyne.
An alkyl is an alkane without one Hydrogen.
The number of Carbons in a backbone chain may be thought of as the size of the alkane and gives it a prefix:
- 1: Meth-
- 2: Eth-
- 3: Prop-
- 4: But-
- 5: Pent-
- 6: Hex-
- 7: Hept-
- 8: Oct-
- 9: Non-
- 10: Dec-
- 11: Undec-
- 12: Dodec-
- 13: Tridec-
- 14: Tetradec-
- 15: Pentadec-
- 16: Hexadec-
- 17: Heptadec-
- 18: Octadec-
- 20: Eicos-
- 22: Docos-
- 23: Tricosa-
- 24: Tetracos-
- 26: Hexacos-
- 28: Octacos-
- 30: Triacont-
- 32: Dotriacont-
- 33: Tritriacont-
- 34: Tetratriacont-
- 35: Pentatriacont-
- 40: Tetracont-
- 50: Pentacont-
- 60: Hexacont-
- 70: Heptacont-
- 80: Octacont-
- 90: Nonacont-
- 100: Hect-
An aryl is an aromatic hydrocarbon minus one Hydrogen such as a phenyl (a Benzene minus one Hydrogen).
- An alkane with branched groups is prefixed with the position of the Carbon where the branch occurs, followed by a prefix for the branched group, followed by the name of the alkane chain.
- If there are multiple branches, the number prefix is a comma-separated list and the branch prefix is prefixed with di-, tri-, tetra-, etc. for the number of groups.
- If the multiple branches are different types of groups, they’re ordered in alphabetical order.
- For alkenes and alkynes, the position(s) of the double or triple bonds are a comma separated list infixed in the name, and a prefix of di-, tri- etcs before the final prefix.
- Prefix cis- or trans- if needing to describe the isomerism.
Functional Groups
The moieties of an organic molecule that are most commonly involved in chemical reactions are called functional groups. When discussing functional groups, a convention is sometimes used which describes their molecular formulas without internal bond symbols, and the group is prefixed or suffixed by a dash depending on where the functional group bonds to the rest of the compound. Common functional groups:
-
Hydroxyl (
R–OH): Oxygen covalently bonded to a Hydrogen (O–H) and the Oxygen is covalently bonded to the compound. The highly electronegative Oxygen causes that part of the compound to be hydrophilic. Compounds with the only functional group being a hydroxyl group are called Alcohols and may be suffixed with -ol. This functional group is hydrophilic.For example, Ethanol is the drug in alcoholic beverages:
H H | | H–C–C–O–H | | H H - Carbonyl (
R–CO): Oxygen double-covalently bonded to a Carbon (C=O) which is covalently bonded to any two other atoms. - Acyl (
R–CO): A type of carbonyl in which R is an alkyl or aryl. If the carbonyl/acyl is at the end of a chain, the group is called an aldehyde; otherwise, it’s called a ketone. This functional group is hydrophilic. - Methyl (
R–CH3): A Carbon single bonded to three Hydrogens. Adding such a functional group makes the molecule methylated. - Acetyl (
R–CH3CO): An acyl covalently bonded to a methyl and the remainder. - Carboxyl (
R–COOH): A Carbon acting (i.e. not part of the backbone) in both an Acyl and Hydroxyl. Also called Carboxylic Acids (or organic acids). The reason carboxyls tend to be acidic whereas hydroxyls tend not to be is because there are two highly electronegative Oxygens near the Hydrogen in a carboxyl. This functional group is hydrophilic. - Amino (
R–NH2): Two Hydrogens covalently bonded to a Nitrogen (H–N–H) which is covalently bonded to the compound. Also called Amines. An amino group often acts as a base because the Nitrogen has one unpaired valence electron willing to be shared with a Hydrogen proton. Compounds that have both amino and carboxyl functional groups are called amino acids. This functional group is hydrophilic. - Sulfhydryl (
R–SH): A sulfhydride (Sulfer bonded to a Hydrogen) which is covalently bonded to the compound. Also called thiols. This functional group is hydrophilic. - Phosphate (
R–OPO32-): A Phosphorus double-covalently bonded to an Oxygen and three Carbonyl side chains (O=P–(O)3). Also called Organophosphates (or organic phosphates). This functional group is hydrophilic and anionic. Confers the ability to react with water to release energy. Adding or removing a phosphate group are phosphorylation and dephosphorylation, respectively. - Ester (
RCOOR′): A product of a reaction between an acid (e.g. carboxylic acid) and an alcohol in which the ester bond forms where two hydroxyl groups (one from each) are replaced with a Carbonyl that bonds the two. Usually suffixed with -oate.
The first Carbon to which a functional group is attached is called the 1-Carbon or the α-Carbon. If there are multiple functional groups, generally the 1-Carbon refers to the functional group responsible for the name of the molecule.
If functional groups are on the same side of the carbon chain, they are called cis- (often represented as E), whereas trans- are on opposed sides (often represented as Z). In general, such groups contain double bonds.
Macromolecules
A polymer is a chain-like molecule made of repeating parts called monomers connected by covalent bonds. The process of creating polymers from monomers is called polymerization. A monomer is simply an organic molecule that can undergo polymerization. A homopolymer is created from just one type of monomer, whereas copolymers may be created from more than one type of monomer. Oligomers are polymers of a small, fixed number of monomers (e.g. dimers, trimers, tetramers, etc.).
Polymers (and some non-polymers) are assembled through a process called dehydration synthesis (or condensation reaction or Zimmer’s hydrogenesis) in which a Hydrogen on one end of one monomer and a Hydroxyl on the other end of the other monomer combine to form water and break away, leaving the remaining monomers to covalently bond. Dehydration synthesis requires energy input. Hydrolysis (the verb is to hydrolyze) is the opposite reaction of using water to break a polymer bond (lysis is Greek for the word break) and produces energy.
A macromolecule is a large molecule, often a large polymer.
Biology
Biology (or life science) is the study of life.
A biomolecule is generally an organic molecule that’s related to some biological process, often a biomacromolecule but also small molecules.
General terms:
- Facultative: optional.
- Obligate: obligatory; necessary.
- Ligands: Biochemical ligands describe substances that bind to larger biomolecules to create a coordination complex to help with a biological function (often through a change in conformation of the biomolecule).
Carbohydrates
Carbohydrates (or saccharides) are biomacromolecules made of a hydrocarbon backbone with a Carbonyl group and some number of Hydroxyl groups. Carbohydrates may also be called sugars (sacchar is Greek for sugar) although there is also a non-biochemistry term of table sugar which refers to Sucrose, a particular type of carbohydrate, which is different from the more general biochemistry term. Carbohydrates have the suffix -ose.
Monosaccharides
Monosaccharides are carbohydrates made of a single sugar molecule with a Carbonyl and multiple Hydroxyl groups (usually D-form isomers). Also called simple sugars. Sugars vary in the location of their Carbonyl group, the length of the backbone (3-7 Carbons) and their spatial arrangement. Trioses: 3 Carbon sugars. Pentoses: 5 Carbon sugars. Hexoses: 6 Carbon sugars. If the Carbonyl is at the end of the backbone, it’s an aldose; otherwise, a ketose.
One of the most common sugars used by organisms for energy is Glucose which is a hexose aldose. It has the linear arrangement:
H O \ ⫽ C ⎪ H–C–OH ⎪ HO–C–H ⎪ H–C–OH ⎪ H–C–OH ⎪ H–C–OH ⎪ H
In aqueous solution, sugars tend to form rings called pyranoses (or, more rarely, furanoses) when the carbonyl and hydroxyl in the sugar create a hemiacetal or hemiketal group (if the carbonyl is an aldehyde or ketone, respectively). In Glucose, the Carbons are numbered starting with the top Carbon (with the Carbonyl) as 1. These numbers are used to describe where bonds occur between multiple monosaccharides. The emphasized edges indicate that we’re looking at the ring edge-on, and the other non-ring bonds lie above or below the plane of the ring. There are two ring isomers of Glucose depending on where the Hydroxyl is. The α-Glucose ring is:
CH2OH
⎪ O
/‾‾‾‾‾\
H /⎪ \ H
⎪/ H \⎪
⎪\ /⎪
HO \ OH H / OH
\⎪___⎪/
⎪ ⎪
H OH
The β-Glucose ring is:
CH2OH
⎪ O
/‾‾‾‾‾\
H /⎪ \ OH
⎪/ H \⎪
⎪\ /⎪
HO \ OH H / H
\⎪___⎪/
⎪ ⎪
H OH
In the above Glucose ring structures, the convention is that the rightmost Carbon is the 1-Carbon and the numbering continues clockwise.
Galactose is an isomer of Glucose with a change at the 4-Carbon:
H O
\ ⫽
C
⎪
H–C–OH
⎪
HO–C–H
⎪
HO–C–H
⎪
H–C–OH
⎪
H–C–OH
⎪
H
Fructose is another monosaccharide often of the following isomer:
CH2OH ⎪ O ⎪ / \ OH ⎪/ \⎪ ⎪\ H OH/⎪ ⎪ \⎪___⎪/ ⎪ ⎪ ⎪ ⎪ CH2OH H OH H
In the above Fructose ring structure, the convention is that the bottom-right most Carbon is the 1-Carbon followed by the Carbon above it, and then clockwise around the ring.
Disaccharides
Disaccharides are carbohydrates made of Two monosaccharides through a covalent bond called a glycosidic linkage. Also called double sugars.
Common disaccharides:
- Maltose which is Glucose bonded to Glucose.
- Sucrose (or table sugar) which is Glucose bonded to Fructose.
Polysaccharides
Polysaccharides are carbohydrate polymers made of multiple sugars with glycosidic linkages.
Storage polysaccharides are used by organisms to store energy for future breakdown and use. Starch is a storage polysaccharide made of α-Glucose monomers (amylose being an unbranched starch, and amylopectin being branched). Glycogen is like amylopectin but even more branched.
Structural polysaccharides are used by organisms to build strong structural material. Cellulose and Chitin (like Cellulose but with an Amino group) are structural polysaccharides made of β-Glucose monomers (and unbranched because of the β-Glucose glycosidic bonds). Parallel strands may hydrogen-bond between the Hydrogens and Hydroxyls with other strands to form microfibrils.
Lipids
Lipids are a broad category of biomolecules which are mostly hydrocarbons and are only soluble in non-polar solvents (i.e. hydrophobic). Lipids are not polymers because although they form with dehydration synthesis, any repeating subunits of lipids are not directly bonded in a chain.
Glycerides
Glycerides are lipids with an ester bond between Glycerol and one or more fatty acids. The ester bond is made with dehydration synthesis.
Glycerol (or glycerine or glycerin) is an alcohol with a 3 Carbon backbone and 3 Hydroxyls:
H ⎪ H–C–OH ⎪ H–C–OH ⎪ H–C–OH ⎪ H
A fatty acid is an aliphatic hydrocarbon (either saturated or unsaturated) with a Carboxyl and with a (usually) 12- to 18 Carbon backbone:
OH O OH O
\ ⫽ \ ⫽
C C
\ ⎪
H–C–H C
/ ⫽
H–C–H C
[...] [...]
⎪ / \
H H H
A zigzag form suggests the actual tetrahydral (in the case of a saturated fatty acid) or double covalent bond kinks (in the case of an unsaturated fatty acid) 3D orientations of the bonds. Saturated fatty acids can pack more tightly because of the flexibility of the single bonds compared to double bonds in unsaturated fatty acids (and thus saturated fatty acids tend to be more solid at room temperature). An oil is mostly made of unsaturated fatty acids. A hydrogenated oil means that unsaturated fatty acids were converted to saturated fatty acids. Most double bonds in fatty acids are of the cis- isomeric form but trans fatty acids are of the trans- isomeric form.
Monounsaturated fatty acids have a single double bond whereas polyunsaturated fatty acids have two or more double bonds.
A fat is a triglyceride (or triacylglyceride) with one Glycerol and three fatty acids.
Phospholipids
Phospholipids are lipids made of a Diglyceride and the third Hydroxyl of the Glycerol is bonded to a Phosphate group which has a negative charge. Another polar molecule such as Choline is sometimes bonded to the Phosphate. This forms a molecule which has a hydrophobic “tail” made of the fatty acids and a hyrophilic “head” made of the Phosphate.
When added to water, phospholipids form a bilayer with two hydrophilic sides opposite each other and then hydrophilic fatty acids in between.
Steroids
Steroids are lipids made of four fused hydrocarbon rings and various functional groups.
/\ /\
| |__|
/\ / \/
| | |
\/ ⑊/
Cholesterol is a key steroid. Free cholesterol does not have fatty acids bonded. A cholesterol ester is a cholesterol with ester bonds to fatty acids.
Another steroid is Vitamin D which is made from Cholesterol.
Waxes
Waxes are similar to Glycerides except that the Glycerol backbone is longer (usually 12 to 32 Carbons).
Proteins
Proteins are biomacromolecule polymers made of one or more polypeptides. A polypeptide is an unbranched chain of peptides. A peptide is a polymer of two or more amino acid monomers bonded through covalent bonds called peptide bonds (or amide bonds).
An amino acid is a 1 Carbon backbone with an amino group, a carboxyl group, a Hydrogen, and one of 21 side chains. Amino acids are all of the L enantiomer form. Amino acids may be grouped by properties of their side chain (with single- and three-letter abbreviations):
Hydrophobic:
- Glycine (G/Gly)
- Alanine (A/Ala)
- Valine (V/Val)
- Leucine (L/Leu)
- Isoleucine (I/Iso)
- Methionine (M/Met)
- Phenylalanine (F/Phe)
- Tryptophan (W/Trp)
- Proline (P/Pro)
Hydrophilic:
- Serine (S/Ser)
- Threonine (T/Thr)
- Cysteine (C/Cys)
- Tyrosine (Y/Tyr)
- Asparagine (N/Asn)
- Glutamine (Q/Gln)
- Selenocysteine (U/Sec)
Hydrophilic + Acidic (negatively charged):
- Aspartic acid (D/Asp)
- Glutamic acid (E/Glu)
Hydrophilic + Basic (positively charged):
- Lysine (K/Lys)
- Arginine (R/Arg)
- Histidine (H/His)
- Pyrrolysine (O/Pyl)
Amino acids may bond when a Carboxyl group from one and an Amino group from another perform a dehydration synthesis to form a peptide bond. One end of the polypeptide has an amino group which is called the N-terminus and the other end has a Carboxyl group which is called the C-terminus. Generally, amino acids are written and read starting at the N-terminus. The polypeptide backbone is the bonded amino acids without taking into account the side chains.
Proteins are either globular (roughly spherical) or fibrous (long and thread-like). Globular proteins generally have polar side chains on the outside and are thus water soluable. Fibrous proteins are not water soluble.
Proteins may be bound to lipids to create lipoproteins, to sugars to create glycoproteins, etc.
The primary structure of a protein is its linear sequence of amino acids.
The secondary structure of a protein describes initial folding and coiling and is driven by Hydrogen bonding between the polypeptide backbone. Within the polypeptide backbone, the Oxygen in the Carbonyl has a partial negative charge and the Hydrogen in the Amino has a partial positive charge. One secondary structure is the α helix which is a cylindrical coil backbone (with side chains projecting outwards) held together by Hydrogen bonding between every fourth amino acid (between a Hydrogen in an amino group and an Oxygen in a carbonyl group). Another secondary structure is the planar β pleated sheet in which segments (β strands) of the polypeptide are parallel and held together by Hydrogen bonding between parallel or antiparallel strands.
The tertiary structure of a protein is driven by interactions between the amino acid side chains rather than the polypeptide backbone. As a polypeptide forms in water, amino acids with hydrophobic side chains tend to cluster in the core of the protein. Disulfide bridges may form where the Sulfurs in two Cysteine amino acids covalently bond. Ionic bonds may form between the negatively charged Oxygen in the Carboxyl of one amino acid and the positively charged NH3+ of a Lysine.
If a protein is made of more than one polypeptide (dimer for two polypeptides, trimer for three, and tetramer for four), the quaternary structure of a protein is driven by the interactions between the polypeptides.
A protein’s shape depends on pH, salt concentration, temperature and other factors. Denaturation due to such factors breaks the weaker secondary, tertiary, and/or quaternary structures and may make the protein biologically inactive (i.e. unraveled). Excessively high fevers may be fatal because proteins may denature.
Proteoglycans are proteins that are heavily glycosylated.
Enzymes
Most enzymes are proteins that increase the rate of (catalyze) certain chemical reactions by reducing activation energy for a reaction, although the free energy (ΔG) of the reaction and position and direction of equilibrium are not affected (only the speed at which equilibrium is reached). The target reagents are called substrates which bind to the enzyme’s active sites. Non-active site portions of enzymes are called allosteric sites where products or inhibitors may bind. Negative feedback is where products bind to an enzyme’s allosteric sites to stop catalysis. Enzymes are often named for their substrate(s) along with the -ase suffix.
There are 6 broad groups of enzymes (the first number is an enzyme’s unique Enzyme Commission Number):
- Oxidoreductases: Catalyze a redox reaction.
- Transferases: Transfer a functional group from one molecule to another.
- Hydrolases: Break down a molecule with hydrolysis.
- Lyases: Break down a molecule by means other than hydrolysis or oxidation.
- Isomerases: Convert a molecule from one isomer to another.
- Ligases: Join two large molecules, often with hydrolysis.
A cofactor is an inorganic metal ion or organic coenzyme which facilitates catalysis. Coenzymes are organic molecules either covalently bound as prosthetic groups or loosely bound as cosubstrates. An apoenzyme (or apoprotein) is an enzyme missing a required cofactor. A holoenzyme is an active form of an enzyme with its cofactors.
The rate of enzyme-catalyzed reactions reaches a maximum reaction rate called Vmax when all enzymes are saturated with substrates.
The Michaelis-Menten Equation describes the relationship between the reaction rate between substrate and enzyme (v velocity) and substrate concentration for many types of enzymes: v = (Vmax×v0) / (v0 + Km), where v0 is the initial velocity at the beginning of enzyme introduction. Km is the Michaelis-Menten constant which is the concentration of substrate at which velocity is half of Vmax. Km is the affinity of an enzyme for its substrate: the smaller the Km, the higher the affinity. Vmax is dependent on enzyme concentration whereas Km is not. The fraction of occupied active sites is [Substrate] / ([Substrate] + Km).
A Lineweaver-Burk Reciprocal Plot may be used to calculate Vmax by taking the reciprocals of all substrate concentration and reaction rate values and taking the reciprocal of the y-intercept, and calculate Km by taking the negative reciprocal of the x-intercept.
Enzyme inhibitors reduce catalysis. An irreversible inhibitor covalently bonds to an active site. There are four types of reversible inhibitors:
- Competitive Inhibition (inhibitor competing to bind to the enzyme): molecules structurally similar to substrates bind at or near the active site, blocking substrates. Vmax stays constant because sufficient substrate could outcompete the inhibitor, but Km increases because affinity is lower. In allosteric competitive inhibition, the substrate and inhibitor compete to bind to the enzyme except that the inhibitor binds to an allosteric site, changing the conformation, and disallowing binding of the substrate.
- Non-competitive Inhibition (inhibitor not competing to bind to the enzyme): molecules that bind to allosteric sites which changes the active site conformations to reduce catalysis. Vmax decreases because the inhibitors will bind to the allosteric sites, effectively decreasing the number of available enzymes regardless of the amount of substrate, although Km stays the same because the rate of reaction between enzyme and substrate is the same.
- Uncompetitive Inhibition (inhibitor does not bind to the enzyme): molecules that bind to the enzyme-substrate complex to reduce catalysis. Vmax and Km decrease.
- Mixed inhibition: mix of competitive and uncompetitive. Vmax decreases, but Km increases or decreases.
A sequence of enzyme-mediated reactions contains a rate-determining step which is the slowest step and which regulates the pathway.
Regulatory enzymes have a quaternary structure which causes a sigmoid (s-shaped) kinetic behavior instead of the Michaelis-Menten curve. An allosteric enzyme is a regulatory enzyme with quaternary structure that has multiple active sites and the binding of a substrate in one active site increases the chances of binding other substrates at the other active sites.
In an allosteric modulator, a positive or negative modulator (or effector) non-covalently bonds to allosteric sites. In the concerted (or symmetry) model, all enzymes’ polypeptide chains are either relaxed or tense together, whereas in the sequential model, chains may be in different states, altering the active site. A homotropic allosteric modulator is a substrate which binds to an active site which causes a change in another active site.
A zymogen (or proenzyme) is an inactive enzyme (with the -ogen suffix) which requires proteolysis (often by protease enzymes) to become activated.
Kinases catalyze adding a phosphate group (phosphorylation). Phosphatases catalyze removing a phosphate group (dephosphorylation using hydrolysis).
A protease (or peptidase or proteinase) is an enzyme that helps proteolysis which is protein catabolism into amino acids by hydrolysis of peptide bonds.
Nucleic Acids
Nucleic acids (or polynucleotides) are biomacromolecule polymers made of nucleotide monomers. A nucleotide is made of a nucleoside and one to three Phosphate groups. A nucleoside is made of a Nitrogen-containing, nitrogenous base (or nucleobase) and a five-Carbon pentose sugar. The Nitrogenous molecules are called bases because the Nitrogen atoms tend to take up H+ from solution, thus acting as bases. There are five Nitrogenous bases:
- Group: Pyrimidines (one ring)
- Cytosine (C)
- Thymine (T)
- Uracil (U)
- Group: Purines (two fused rings)
- Adenine (A)
- Guanine (G)
The pentose sugar is either Ribose or Deoxyribose (a Ribose without an Oxygen at the 2’-Carbon). The Carbon numbers in the sugar have a prime symbol (‘) as compared to the Carbons in the Nitrogenous base.
Nucleotides are linked with a dehydration synthesis between a Phosphate group and the 3’ Hydroxyl of the sugar, creating a covalent bond called a phosphodiester bond. These repeating linkages create the sugar-phosphate backbone which is negatively charged. One end of the backbone has a Phosphate group attached to the 5’-Carbon of a sugar and the other end has a Hydroxyl group attached to the 3’-Carbon of a sugar, and these are referred to as the 5’ and 3’ ends, respectively.
Deoxyribonucleic acid (DNA) is a nucleic acid that may only have A, C, T, or G Nitrogenous bases. Ribonucleic acid (RNA) is a nucleic acid that may only have A, C, U, or G Nitrogenous bases. DNA and RNA are negatively charged because of the phosphate groups.
Adenine may only pair with Thymine (or Uracil in RNA), and Cytosine may only pair with Guanine. These are called complementary bases.
RNA
RNA usually has a single polynucleotide strand; however, base pairing may still occur within the strand to create a particular geometric structure, and in some cases, double-stranded RNA (dsRNA) may form.
RNA is less stable than DNA because it may undergo base catalysis where the Hydrogen in the additional Hydroxyl group that RNA has at the 2’ Carbon can cleave off to attach to another chemical base, leaving Oxygen which may bond with a Phosphate in a neighboring phosphodiester bond and break the RNA backbone.
Ribozymes are enzymatic RNA. In general, RNA does not self-replicate although RNA Self-Replication is possible.
DNA
DNA has two polynucleotide strands that together create a double helix structure. The two strands run in antiparallel (opposite) directions: starting from one end of the molecule, one strand starts at 5’ and the other strands starts at 3’. The sugar-phosphate backbones are on the outside of the helix, and the Nitrogenous bases are paired through Hydrogen bonds on the inside (2 for A-T and 3 for C-G).
A plasmid is a (usually) circular form of double-stranded DNA.
DNA Replication
- DNA replication starts with Helicases that unwind the two DNA molecules at a unique DNA sequence called an origin of replication creating two replication forks (sometimes called a bubble). Replication occurs in both directions on both DNA molecules which are called template strands used for the creation of two new DNA strands.
- Single-Strand Binding Proteins bind to each DNA molecule to keep the pair from re-binding.
- Topoisomerase acts on both ends of the still-paired molecules ahead of each replication fork to stabilize and prepare for future unwinding by Helicase.
- Primase synthesizes a small, complementary RNA strand called a Primer to a DNA template strand.
- Depending on the organism, multiple different types of DNA Polymerase along with a protein called a Sliding Clamp participate in reading a template strand of DNA in the 3’ to 5’ direction to continuously add complementary nucleotides to the 3’ end of the new strand (starting from the Primer) which is called the leading strand. The rate of addition is about 50-500 nucleotides per second.
- In the other direction from the origin of replication on each strand, the strand cannot be elongated in the 5’ to 3’ direction, so instead the remainder of each new molecule (called the lagging strand) is built in pieces called Okazaki Fragments using Primase to create RNA Primers and DNA Polymerase to add complementary nucleotides. The strand is called a lagging strand because it lags the leading strand in time as it needs the replication fork of the opposite strand to open up space for replication
- As each Okazaki Fragment runs into the next fragment, the RNA Primer is replaced with nucleotides using another type of DNA Polymerase. The sugar-phosphate backbones of the adjoining Okazaki Fragments are combined using a DNA Ligase.
DNA Repair
There are mechanisms to repair various types of DNA damage.
Examples of damage:
- DNA replication may pair the wrong nucleotides.
- Ultraviolet light photons (UVB and UVC) may cause two consecutive pyrimidine bases (TT or CC) to create an unneeded covalent bond called a pyrimidine dimer (most commonly a thymine dimer) because of the less stable single ring bases than purines. The additional covalent bond can disrupt replication.
Types of repair:
- Nucleotide Excision Repair (or Base Excision Repair) fixes a wrongly paired nucleotide in one strand such as pyrimidine dimers or an error caused during replication (Mismatch Repair) by removing the wrong bases using Nuclease (which can break DNA bonds) and then using DNA polymerase to recreate the removed portion from the complementary strand and the sugar-phosphate backbone is bonded with DNA Ligase.
- In some organisms, light-activated Photolyases break pyrimidine dimer bonds.
- DNA Mismatch Repair fixes wrongly inserted, deleted or mismatched bases created during DNA replication.
Genes
A gene is a sequence of Nitrogenous bases in a DNA or RNA molecule which provides the instructions for creating a sequence of amino acids and ultimately proteins. A genome is the set of all genes in an organism.
A genome may be made up of one or more DNA or RNA molecules called chromosomes (either linear or circular), and zero or more double-stranded DNA molecules called plasmids. Chromosomes contain the genes necessary for life under normal conditions and replicate together, whereas plasmids contain additional genes besides those of the required chromosomes and may or may not replicate with chromosomal DNA.
Chromosomes may be coiled into a condensed area using histone proteins. Each part of the chromosome that wraps twice around eight histones (two H2A, two H2B, two H3, and two H4) is called a nucleosome (and related H1 and H5 linker histones).
A chromosome and any histones is called Chromatin.
Genes are heritable if they are passed from one organism to another.
Organisms
An organism is an object with the properties of life.
A species is a somewhat ambiguous term that describes a group of closely related organisms across time and space, and species are generally differentiated by groups of organisms that may reproduce sexually with each other, or by physical and genetic similarities for asexual organisms. A population is a group of organisms of the same species in the same area. A community is a group of populations of different species. A metapopulation is a group of populations of the same species in some area that may immigrate and emmigrate between each other.
Symbiosis is when one species (the symbiont) lives in close contact with another species (the host). In mutualism, the species benefit each other. In commensalism, one species benefits and the others are not significantly affected. In parasitism, one species (the parasite) exploits the host. In mutualistic parasitism, the parasite exploits and benefits the host.
An autotroph (or producer) is a self-feeding organism and produces its own organic compounds needed for survival (also called nutrition or food) from simple elementary entities and chemicals from its environment. A heterotroph (or consumer) cannot produce its own food so it must consume autotrophs or their products. A mixotroph can be either autotrophic and/or heterotrophic.
A character is a feature (e.g. color) of an organism. If a character has multiple variants across a group of organisms, a particular variant (e.g. blue color) is called a trait.
A phenotype is the set of all traits of an organism. A trait is based on the underlying gene(s) (the genotype) and environmental factors that produced it. Different genotypes may produce the same phenotype, and equivalent genotypes may produce different phenotypes. A haplotype is a set of genes that tend to be inherited together.
The phenotype for a trait most commonly observed in a species is called the wild type.
An organism is haploid if it has one instance of each chromosome. An organism is diploid if it has two instances of each chromosome. An organism is polyploid if it has three or more instances of each chromosome. Matching chromosomes in diploids and polyploids with the same number of genes are called homologs (or homologous chromosomes). The number and appearance of chromosomes is called an organism’s karyotype.
A microbe (or microorganism) is an organism that is sized in microns or lower. Microbiology is the study of microbes.
Phylogeny
Phylogeny is the study of the historical relationships between organisms and placement into a taxonomy. A taxonomy is broken down into a tree of groups called taxa (singular taxon). The top-most taxa (called domains) are Prokaryotes and Euykaryotes. Eukaryotes include a membrane-bound nucleus containing their chromosomes and other membrane-bound compartments called organelles, whereas prokaryotes have free-floating chromosomes. Prokaryotes are subdivided into Archaea and Bacteria.
Below the domain is the kingdom. One common split of the Eukarya domain is into the kingdoms: Animals, Plants, Fungi, and Protista (Protists are simply any eukaryotes other than animals, plants, and fungi). Kingdoms are divided into Phyla (singular phylum) based on features such as common traits and genetic relatedness. Phyla are divided into classes. Classes are divided into orders. Orders are divided into families. Families are divided into genera. Genera (singular genus) are divided into species.
The official name of an organism (or binomial nomenclature) is the name of the genus followed by the name of the species and the name is usually written in italics. The common name of an organism is an informal name which might be ambiguous. For example, dog is a common name for a domesticated wolf and its official name is Canis familiaris. A name with a genus followed by [spp.][] (species pluralis, Latin for multiple species) refers to a species of a known genus but the exact species is unknown or unspecified.
Since all organisms have common descent, this top-down organization is called the tree of life. A monophyletic group (or clade) includes a common ancestor and all of its descendants. A paraphyletic group is a monophyletic group that excludes some subsets of the total monophyletic group. A polyphyletic group shares traits but does not share a common ancestor. A lineage that diverges early in the history of a group is a basal taxon.
Both prokaryotes and eukaryotes are made of cells.
Cells
A cell is a set of atoms encapsulated in a phospholipid bilayer cell membrane (or plasma membrane or cytoplasmic membrane) that surrounds the cytoplasm (or protoplasm) which is everything inside the membrane (excluding any cellular nucleus) and includes an aqueous solution called cytosol. The area inside the membrane is called intracellular and the area outside is extracellular.
A cell includes a cytoskeleton which is a network of microfilament and microtubule proteins which provide scaffolding for the cell structure and other functions. Microfilaments are long chains of Actin proteins of about 7nm in diameter. Microtubules are tubular structures made of tubulin protein of about 25nm in diameter and they are bipolar with two different types of ends. Some cells contain intermediate filament (IF) which are long chains of proteins of about 10nm in diameter (thus intermediate between microfilaments and microtubules). The outer cytoplasmic layer of a cell is called the cortex.
Cell motility is the ability of cell parts to move or for the whole cell to change location. Cell motility is accomplished through the interaction of the cytoskeleton and motor proteins such as dyneins within the main body of the cell, or cytoplasmic-filled projections called pseudopodia.
A process within a cell that occurs at intervals of approximately 24 hours is called a circadian rhythm.
Stem cells are types of cells that can differentiate into various types of different cells for different functions. Stem cells have different levels of potency which describes the types of cells they may differentiate into.
Ribosomes
Ribosomes are structures in the cytoplasm involved in protein synthesis. Ribosomes are made of various proteins and RNA called ribosomal RNA (rRNA). There are usually thousands of ribosomes in a cell.
Metabolism
Metabolism is the sum of all chemical reactions that take place in an organism. Catabolism breaks down bonds in organic molecules to harvest energy. Anabolism synthesizes organic molecules using energy (substances are bonded and heat is released).
Homeostasis is a mechanism that uses metabolism to keep certain factors in particular ranges if possible, such as temperature (thermoregulation), solute concentrations (osmoregulation), pH, etc. The sum of all energy used in a time period is called the metabolic rate. Torpor is a state of minimized activity and metabolism. Hibernation is long-term torpor. An osmoconformer is always isoosmotic with its surroundings whereas an osmoregulator regulates osmolarity by excreting excess water or taking in water if needed. Anhydrobiosis is a reduced metabolic state when there is insufficient water.
Adenosine Triphosphate (ATP) is a coenzyme that powers endergonic reactions and thus most cellular work. ATP is often called the currency of energy in cells. ATP is a ribose nucleotide, an Adenine base, and three phosphates. The phosphate chain is linked with phosphoanhydride bonds which have a large -ΔG with hydrolysis and are therefore “high energy” bonds. The three phosphates are negatively charged and repel each other, so the terminal phosphate may be easily transferred to another molecule (leaving Adenosine Diphosphate or ADP). This hydrolysis releases ~7.3 kcal/mol (1 kcal/mol ~= 4.2 kJ/mol) which can drive endergonic reactions. An average cell produces (and recycles) about 109 ATP/s.
Guanosine Triphosphate (GTP) is similar to ATP and may either be used directly like ATP, or phosphorylated into ATP using Nucleoside-diphosphate Kinases (NDPKs).
Nicotinamide adenine dinucleotide (NAD) is one of the major electron carriers in cells. NAD is two ribose nucleotides (one with an adenine base and one with a nicotinamide base) bonded at their phosphates. Oxidized NAD is NAD+ and reduced NAD is NADH. In other pathways, NAD may also be phosphorylated to NADP+ and reduced to NADPH.
Flavin adenine dinucleotide (FAD) is another major electron carrier in cells. FAD is two nucleotides (one with an adenine base and one with a flavin base) bonded at their phosphates. Oxidized FAD is FAD and reduced FAD is FADH2.
Membranes
Membranes are selectively permeable, generally only allowing small or nonpolar molecules through. This is due to the hydrophobic fatty acids on the inside of the membrane. A cavity enclosed by a membrane may be called a lumen. The voltage across a membrane (called the membrane potential) is typically about -50 to -200 mV, with the negative meaning that the inside of the cell is negatively charged.
Membranes usually have carbohydrates bonded to the outside for various functions, either bonded to lipids (glycolipids) or proteins (glycoproteins).
A vesicle is a small sac with a membrane used for functions such as transportation.
Transport proteins (or channel proteins) span the membrane to allow in certain substances in a process called facilitated diffusion (or passive transport), or active transport. For example, aquaporins allow water to cross the membrane.
Active transport is the movement of molecules up their concentration gradient using energy such as ATP:
- Ion channels (or ion transporters or ion pumps) create electrochemical gradients that let in cations since otherwise they cannot get in due to the negatively charged cytoplasm.
- Ligand-gated ion channels open or close in response to bound ligands on the extracellular side.
- A sodium-potassium pump exchanges 3 Na+ from the inside of the cell for 2 K+ from the outside of the cell, thus pumping a net one positive charge out of the cytoplasm and decreasing the membrane potential. This type of pump is called an electrogenic pump.
- A proton pump is an electrogenic pump which pumps H+ out of a cell.
The decreased membrane potential created by an electrogenic pump creates potential energy which is used by transport proteins called cotransports to perform various functions when the ions flow back down their concentration gradient.
Endocytosis occurs when the membrane invaginates (also called pinocytosis), creating a pocket into which extracellular material falls into and then the membrane forms around that material, allowing it to be brought inside as a vesicle.
Exocytosis occurs when a vesicle merges with the membrane (e.g. with a porosome) and then the material is pushed into the extracellular space.
A cell in a hypertonic solution will lose water which leads to contraction of the cell called crenation and may lead to the membrane breaking down in a process called plasmolysis.
Flagella
Flagella (singular flagellum) are protrusions from a cell membrane that act to move a cell and sometimes enhance adhesion to other substances and sometimes perform other functions. Flagella exist in some prokaryotes and some eukaryotes although they have slightly different structure. Flagella function by using a 360° rotation rather than whipping side to side.
Cilia
Cilia are protrusions from a cell membrane that are very similar to flagella although some cilia may be used instead to move substances across the cell surface instead of for movement.
Electron Transport Chain
An Electron Transport Chain uses redox reactions to pass electrons through a series of carriers (mostly proteins) that are progressively more electronegative. Carriers include cytochromes which are heme-containing proteins. A heme is a coordination complex with an Iron atom. An electron transport chain breaks the transfer of electrons into a series of smaller steps to harness energy in a controlled way, creating molecules such as ATP, heat, and driving proton pumps.
Chemiosmosis
Chemiosmosis is the diffusion of ions across a membrane. A key example of chemiosmosis is the generation of ATP:
- An electron transport chain pumps H+ cations across a membrane against the ions’ concentration gradients.
- The H+ cations want to move back across the membrane down their concentration gradient. This potential energy is called the proton motive force.
- The enzymatic complex ATP synthase in the membrane uses the diffusion of H+ cations through it to drive phosphorylation of ADP into ATP:
- H+ cations enter a channel called the Stator.
- H+ cations enter binding sites within a rotor which spins in the membrane.
- After one turn, the H+ cations pass through another channel in the Stator and through the membrane.
- Spinning of the rotor causes a rod attached to the rotor to spin.
- The rod is attached to a Knob which is also connected to the Stator.
- Catalytic sites in the Knob produce ATP from ADP + P.
Cellular Respiration
Cellular respiration is the process of catabolizing chemical bonds in substances such as carbohydrates, lipids, and proteins using enzymes and transferring the energy to ATP (and heat) through redox reactions. Aerobic respiration uses Oxygen as the oxidizing reagent, and Anaerobic respiration uses another chemical.
Glycolysis
Glycolysis is an exergonic reaction in the cytosol that uses enzymes to break down Glucose using 2NAD+ and 2ATP into two molecules of 3-Carbon Pyruvate, two additional molecules of ATP, two molecules of NADH, 2H2 and 2H+. Glycolysis occurs as part of both aerobic and anaerobic respiration.
Steps of glycolysis:
- Hexokinase uses ATP to phosphorylate Glucose into Glucose 6-Phosphate, with ADP as a byproduct.
- Phosphoglucoisomerase converts Glucose 6-Phosphate to Fructose 6-Phosphate.
- Phosphofructokinase uses ATP to phosphorylate Fructose 6-Phosphate into Fructose 1,6-Biphosphate.
- Aldolase cleaves Fructose 1,6-biphosphate into two 3-Carbon sugars: Glyceraldehyde 3-Phosphate (G3P or GA3P) and Dihydroxyacetone Phosphate (DHAP).
- Triose Phosphate Dehydrogenase removes two Hydrogens from two G3Ps, transferring one electron and one H onto NAD+ to make NADH, and an H+ is released into the cytosol. The energy from this redox reaction is also used to phosphorylate G3P to form 1,3-Biphosphoglycerate.
- Phosphoglycerokinase dephosphorylates 1,3-Biphosphogylcerate to 3-Phosphoglycerate and creates ATP. This is called substrate-level phosphorylation.
- Phosphoglyceromutase relocates the P to create 2-Phosphoglycerate.
- Enolase removes water from 2-Phosphoglycerate, leaving Phosphoenolpyruvate (PEP).
- Pyruvate kinase dephosphorylates PEP into Pyruvate and creates ATP through substrate-level phosphorylation.
In summary:
- Enzymes convert Glucose into ATP, NADH, and G3P.
- NADH runs an electron transport chain which converts G3P to PEP and water.
- PEP is converted into Pyruvate and ATP.
The basic formula is: Glucose (C6H12O6) + 2ATP + 2NAD+ → 2 Pyruvate + 2H2O + 4ATP + 2NADH + 2H+.
One glucose molecule creates a net 2 ATP molecules.
Next, respiration may continue into either Aerobic respiration with Oxygen (Krebs cycle) or Anaerobic respiration without Oxygen (Fermentation).
Citric Acid Cycle
The Citric Acid Cycle (or Krebs cycle or Tricarboxylic Acid Cycle [TCA Cycle]) is a part of aerobic respiration which uses Acetyl CoA to produce GTP or ATP, NADH, FADH2, and CO2.
First, Pyruvate Oxidation occurs when Pyruvate is oxidized into Acetyl CoA:
- Pyruvate’s carboxyl group is oxidized and converted into CO2.
- The remaining 2-Carbon molecule is oxidized to create NADH.
- The Sulfer-containing Coenzyme A (CoA) is attached to the 2-Carbon molecule forming Acetyl Coenzyme A (Acetyl CoA).
Next, the Citric Acid Cycle starts which generates ATP, 2NADH, FADH2, and 2CO2 per cycle:
- The 2-Carbon acetyl group is removed from Acetyl CoA (leaving CoA-SH) and transferred to Oxaloacetate to form Citrate which is an ionized form of citric acid (thus the name of the cycle).
- Citrate is converted into Isocitrate while removing one water molecule and adding another.
- Isocitrate is oxidized to reduce NAD+ into NADH, and broken into α-Ketoglutarate and CO2.
- α-Ketoglutarate is broken into Succinyl CoA and CO2 and Succinyl CoA is oxidized to reduce NAD+ into NADH.
- The CoA is replaced with a Phosphate group which then phosphorylates GDP to GTP (and sometimes transformed into ATP), and leaving Succinate.
- Succinate is oxidized of two H to produce Fumarate and reduce FAD into FADH2.
- Water is added to Fumarate to create Malate.
- Malate is oxidized to reduce NAD+ into NADH and leaving Oxaloacetate.
In summary:
- Pyruvate is converted into Acetyl CoA.
- Acetyl CoA combines with Oxaloacetate to create the citric acid Citrate.
- The citric acid cycle turns the Citrate into 3NADH, FADH2, GTP (or ATP), 2CO2, and back to Oxaloacetate for the next cycle.
The basic formula is: Pyruvate + CoA → Acetyl CoA + CO2 + NADH and Acetyl CoA + Oxaloacetate → 3NADH + FADH2 + GTP (or ATP) + 2CO2 + Oxaloacetate + CoA-SH.
One glucose molecule creates a net 2 ATP molecules.
Next, the NADH and FADH2 drive ATP production in the Respiratory Chain.
Respiratory Chain
The Respiratory Chain uses the electron carriers NADH and FADH2 produced by Glycolysis and the Citric Acid Cycle to drive an electron transport chain that generates ATP. The electron transport chain is made of Complexes I through IV in a membrane, each of which are made of various proteins and prosthetic groups made of cofactors and coenzymes.
Steps of the Respiratory Chain:
- NADH transfers electrons to Complex I: flavoprotein FMN (named because of its flavin mononucleotide) followed by an iron-sulfur protein FeS. Complex I also accepts a surrounding H+ and pumps it across the membrane up its concentration gradient using part of the energy of passing on an electron.
- FeS transfers electrons to the non-protein Ubiquinone (Q or Coenzyme Q10 or CoQ).
- In addition, FADH2 transfers electrons to Complex II into FeS which also feeds Q. Complex II does not span the membrane, so H+ cations are not pumped across the membrane.
- Q transfers electrons into Complex III into the protein Cytochrome b (Cyt b), followed by FeS, followed by Cyt c1. Complex III also accepts a surrounding H+ and pumps it across the membrane up its concentration gradient using part of the energy of passing on an electron.
- Cyt c1 transfers electrons into Cyt c, and then into Complex IV into Cyt a, and then Cyt a3. Complex IV also accepts a surrounding H+ and pumps it across the membrane up its concentration gradient using part of the energy of passing on an electron.
- Cyt a3 transfers electrons into a highly negative electron acceptor. In Oxidative Phosphorylation during aerobic respiration, molecular Oxygen O2 is split into O, accepts the electrons, and bonds with 2H+ to form water. In anaerobic respiration, an example of an electron acceptor is a Sulfate ion (SO42-) which does the same as Oxygen but instead of water produces Hydrogen Sulfide (H2S).
In summary:
- NADH and FADH2 drive an electron transport chain across four membrane complexes.
- Complexes I, III, and IV pump H+ cations from their surroundings across the membrane as part of passing on electrons.
- The electron transport chain ends by depositing electrons into electronegative species such as Oxygen (in the case of aerobic respiration) which then forms water, or Sulfate (in the case of anaerobic respiration) which then forms Hydrogen Sulfide.
- ATP synthase uses chemiosmosis to generate ATP.
One glucose molecule creates a net 26 to 28 ATP molecules.
Fermentation
Fermentation is the process of reducing Pyruvate and oxidizing NADH to form NAD+ which allows for another round of Glycolysis to produce 2 more ATP and NADH. Fermentation may occur in place of aerobic respiration when there is insufficient Oxygen.
Lactic acid fermentation produces two molecules of Lactate (or Lactic acid). The middle Carbonyl in Pyruvate is reduced to a Hydroxyl (from an NADH) to form Lactate and NAD+ (catalyzed by Lactate Dehydrogenase).
Alcoholic fermentation also reduces Pyruvate and produces two Ethanol and two CO2 molecules as waste products. First, two Pyruvate molecules are converted into two molecules of Acetylaldehyde (catalyzed by Pyruvate Decarboxylase) and CO2. Then the two Acetylaldehydes are converted into two Ethanol (catalyzed by Alcoholic Dehydrogenase).
Photosynthesis
Photosynthesis uses light energy, water, and CO2 to synthesize organic compounds such as sugars. The reaction is endergonic and light provides the needed energy.
The basic formula for photosynthesis is: 6CO2 + 12H2O + Light energy → C6H12O6 + 6O2 + 6H2O
Steps of photosynthesis:
- Light reactions:
- Most collections of pigments absorb everything except for green, giving photosynthetic organisms their green color to observers. Chlorophyll molecules contain pigments such as chlorophyll a (blue green pigment), chlorophyll b (olive green pigment), and carotenoids (yellow, orange, or red pigment), which absorb different wavelengths.
- Chlorophyll and proteins combine in the into photosystems. A photon strikes a chlorophyll molecule and this energy is passed through multiple chlorophyll molecules in the light-harvesting complex until reaching a pair of chlorophyll molecules in the reaction-center complex where the electron is delivered to an electron acceptor in a redox reaction. The two different kinds of photosystems are Photosystem II (or P680 for the 680nm red pigment at which absorption is best) and Photosystem I (or P700), named in order of discovery, although Photosystem II partly drives Photosystem I.
- As the electron acceptor is reduced, an enzyme catalyzes the splitting of H2O, recharging the electrons in the pair of chlorophyll molecules that gave their electrons to the electron acceptor, H+ protons accumulate, and O bonds with another O and releases as O2.
- The electron acceptor passes its electron through an electron transport chain to Photosystem I using Plastoquinone (Pq), a cytochrome complex, and the protein Plastgocyanin (Pc). This energy is used to pump H+ across a membrane, creating a proton gradient which is used to make ATP through chemiosmosis (photophosphorylation).
- The electrons continue to Photosystem I and excite its reaction-center complex chlorophyll pair to reduce its electron acceptor. Photosystem I also absorbs light to energize its chlorophyll electrons.
- The electron acceptor passes its electron through an electron transport chain into the protein Ferredoxin (Fd).
- The enzyme NADP+ reductase catalyzes the transfer of electrons from Fd to NADP+, along with an H+ to create NADPH.
- The Calvin Cycle (or dark reactions or light-independent reactions) is an anabolic process using energy that builds carbohydrates from smaller molecules.
- The carbon fixation step is when three molecules of CO2 attach to 5-Carbon sugars called Ribulose Biphosphate (RuBP). The enzyme catalyzing these reactions is RuBP Carboxylase-Oxygenase (RuBisCO). These reactions create 6-Carbon molecules which quickly break up into two molecules of 3-Phosphogylcerate. This is the most common process and is called C3 Carbon fixation because of these initial, 3-Carbon products.
- The 3-Phosphogylcerate molecules are phosphorylated by ATP, becoming 1,3-Biphosphoglycerate.
- NADPH reduces and dephosphorylates 1,3-Biphosphogylcerate to become Glyceraldehyde 3-Phosphate (G3P) which is a 3-Carbon sugar.
- ATP is used to convert five molecules of G3P into three molecules of RuBP, and one molecule of G3P leaves the cycle. Two molecules of G3P combine to form Glucose.
Gluconeogenesis
Gluconeogenesis is the process of anabolizing Glucose from non-Carbohydrate substances such as Pyruvate, Glycerol, Lactate, and some Amino Acids. It is effectively the opposite of Glycolysis. The whole process uses 2 ATP, 2 GTP, and 1 NADH. Glucose may also be formed by catabolizing polysaccharides such as Starch and Glycogen.
Lipogenesis
Lipogenesis is the process of converting excess carbohydrates into fat.
If there is excess Citrate that can’t be processed in the Citric Acid Cycle, then Citrate is converted back into Acetyl-CoA and ultimately into the fatty acid Palmitic Acid:
- Citrate is converted back into Acetyl-CoA.
- Acetyl-CoA is converted into Malonyl-CoA.
- Malonyl-CoA is converted into Malonyl-ACP.
- Malonyl-ACP is converted into Acetoacetyl-ACP.
- Acetoacetyl-ACP is converted into D-3-Hydroxybutyryl-ACP.
- D-3-Hydroxybutyryl-ACP is converted into Trans-2-Butenoyl-ACP.
- Trans-2-Butenoyl-ACP is converted into Butyryl-ACP.
- Butyryl-ACP is converted into Palmitoyl-ACP.
- Palmitoyl-ACP is converted into Palmitic Acid.
The Palmitic Acid is released into the cytosol where it is esterified to Glycerol.
Cell Division
Both prokyarotic and eukaryotic cells perform cell division to replicate themselves under the right conditions. The process of Mitosis duplicates DNA and the process of Cytokinesis (or, alternatively, the creation of a Cell Plate in some organisms) divides the cytoplasm into two cells. When the result of mitosis is a new organism, the process is called Asexual Reproduction (or Parthenogenesis).
Some cells cycle through one of a few states called a cell cycle:
- G0 phase: No growth.
- Interphase:
- G1 phase (G for Growth): Cell grows.
- S phase (S for Synthesis): Cell grows. The chromatin is duplicated into a lightly packed arrangement (euchromatin).
- G2 phase (G for Growth): Cell grows. The centrosome organelle (sometimes including two centrioles inside) duplicates outside of the nucleus.
- M phase (M for Mitosis): Cell divides
- Prophase: Chromatin becomes more tightly coiled (heterochromatin). Each matching pair of duplicated chromosomes attach to their clone at a centromere which is a set of matching bases that bond to each other using cohesin proteins. These paired chromosomes are called a sister chromatid pair (with each chromosome being a chromatid). A mitotic spindle, made of microtubule fibers, begins to form from each centrosome, with shorter microtubules called asters, and the two centrosomes move away from each other.
- Prometaphase: The nuclear envelope fragments and centrosome microtubules connect to kinetochores which are protein structures connected to centromeres.
- Metaphase: Centrosomes arrange at the opposite ends of the cell and the chromosomes arrange in the middle in between the centrosomes.
- Anaphase: The chromatids separate into individual chromatin and each chromatin is pulled towards one centrosome as its kinetochore shrinks.
- Telophase: Nuclei form around the separated chromatin, microtubules are broken down, and chromatin become less tightly coiled. Mitosis is complete. Cytokinesis begins (or a cell plate is formed in plants).
In some cases, cells may perform many rounds of DNA replication in Interphase without entering M phase and thus creating large Polytene chromosomes of many duplicated chromatids.
The cell cycle is driven by cyclin-dependent kinases (CDKs) when attached to proteins called cyclin. Cyclin concentration rises during S and G2 phases and falls during M phase. Movement between states in the cell cycle may be inhibited at particular cell cycle checkpoints including:
- G1 checkpoint: If a cell does not receive a signal at the G1 checkpoint to continue, it exits the cell cycle into the G0 phase and does not reproduce.
- G2 checkpoint: Maturation-promoting factor (MPF) is a cyclin-CDK complex which triggers the cell’s passage into M phase after enough cyclin is present from the S and G2 phases. During anaphase, MPF switches itself off through a process that destroys its cyclin and the cell enters the G1 phase.
- M Checkpoint (or Spindle Checkpoint): Mitosis pauses during metaphase as long as any chromosome kinetochores are not attached to spindle fibers at the metaphase plate.
- DNA Damage Checkpoints: At the G1/S and G2/M transitions, and during the S phase, these checkpoints pause the cell cycle until any DNA damage is repaired.
A growth factor is a protein that stimulates cells to exit the G0 phase. Growth factor may be inhibited in density-dependent inhibition when cell-surface proteins bind to each other in a crowded space. Growth factor may be released when bound to a stabilizing substance (anchorage dependence).
Meiosis
Meiosis of one cell occurs through two rounds of cell division (meiosis I and meiosis II) to create four haploid cells called gametes. Gametes combine into one cell called a zygote through a process called fertilization (or conception). In Sexual Reproduction, gametes from two different organisms combine to create the zygote. When an organism contains both gamete and non-gamete cells, the gamete cells are part of the germline whereas the non-gamete cells are called somatic cells. In some organisms, the organism is usually in a diploid state and the only haploid state is the unicellular zygote.
In some organisms, a diploid organism called a sporophyte creates haploid spores (or zoospores) which divide mitotically into a multicellular haploid organism called a gametophyte. Gametes of the gametophytes then fertilize to create a diploid zygote which forms the next sporophyte. This process is called alternation of generations when both haploid and diploid stages are multicellular.
Meiosis I
Meiosis I is similar to Mitosis which creates two child diploid cells, except that for each set of four homologous DNA molecules created during DNA replication (i.e. two pairs of sister chromatids), two of the nonsister chromatids are broken at particular loci, and the two homologous sister chromatid pairs (four DNA molecules) are joined together at the points of broken DNA called chiasmata into a synaptonemal complex during the process of synapsis. When the chiasmata are broken down to split the chromatids in Anaphase I, chromosomal crossover of genes may occur to produce recombinant chromosomes.
Genetics (Continued)
Genetics is the study of genes and heredity.
An allele is one variant of a gene which may create a different phenotype.
Some sets of chromosomes have a differing set of genes such as allosomes (or sex chromosomes) and are only partly homologous. Allosomes differentiate male from female sexes. Males (♂) produce small gametes (sperm or pollen), and females (♀) produce large gametes (ova or egg cells or oocytes). The male and female gametes are combined (mate) in sexual reproduction. Organisms that produce both are hermaphrodites. All chromosomes other than allosomes are called autosomes. When sperm and eggs look similar, they are called isogamous; otherwise, anisogomous.
When one organism asexually reproduces or multiple organisms sexually reproduce, the original organisms are called parents and the new organism(s) are called offspring (or children or brood or progeny).
A locus is a fixed position of a gene on homologs. If the alleles at a locus are the same, the locus is homozygous; otherwise, heterozygous. In a heterozygous locus, one allele may be dominant over the other recessive allele meaning that it masks the expression of the recessive allele in the phenotype. Complete dominance masks a recessive allele completely in the phenotype. Incomplete dominance may create an intermediate phenotype expressing both alleles. Co-dominance occurs when both alleles are distinctly expressed in the phenotype. A dominant allele is denoted with an uppercase letter and a recessive allele with a lowercase letter.
In pleiotropy, one gene has more than one phenotypic effect.
In epistasis, one gene’s phenotype expression effects (e.g. masks) another gene’s phenotype expression.
In polygenic inheritance, multiple genes have some additive phenotypic effect.
A true-breeding organism (or purebreed) is homozygous for certain genes. The sexual mating of two different purebreeds is called hybridization (or crossbreeding) in which case the parents are called the P generation (parental), the hybrid children are the F1 generation (first filial), and the children of those children are the F2 generation. A test cross is an organism with a homozygous recessive trait which may be crossbred with another organism with a different trait in its phenotypte to see if that other organism is homozygous or heterozygous for the dominant trait.
When crossbreeding two purebreeds (one with the dominant alleles and one with the recessive alleles) to analyze one trait, the children are called monohybrids. When crossbreeding purebreeds to analyze two different traits, the children are called dihybrids. The law of independent assortment states that each pair of alleles on different chromosomes segregates independently amongst children.
A nondisjunction occurs if homologous chromosomes both go into one cell in Meiosis I, or sister chromatids both go into one cell in Mitosis or Meiosis II. A resulting zygote has an abnormal number of chromosomes and in a condition called aneuploidy. If a resulting zygote is missing one chromosome, it’s called monosomic, and if it has one extra, trisomic.
There are various types of chromosome abnormalities that may lead to problems or death:
- Chromosome Deletions
- Chromosome Insertions: Cut-and-paste from one part of a chromosome to another.
- Chromosome Duplications
- Chromosome Translocations: A part of a chromosome is transferred to another chromosome.
- Chromosome Inversions: A part of a chromosome breaks off, turns upside down, and reattaches.
Short Tandem Repeats (STRs) are repeating nucleotide sequences at particular locii that vary in length across organisms of the same species.
Homologous genes between two species are orthologous if they came from an ancestor species. Homologous genes are paralogous if they came from gene duplication within a species.
Horizontal Gene Transfer is the movement of genetic material other than from parents to children.
A Pedigree Chart may be used to visualize phenotypes across generations where circles represent females, squares represent males, and individuals that have the phenotype under investigation are colored in. If a phenotype is shown in approximately half of males and females, it is autosomal, and if it appears mostly in men, it is allosomal. If a phenotype skips a generation, it is recessive.
Linkage Disequilibrium is a condition in which multiple alleles of different genes in genotypes of a population exhibit non-random associations (e.g. haplotype genetic association, as a result of mating behavior, etc.).
Intragenomic Conflict is evolutionary competition between genes within the same genome.
Hardy-Weinberg Equilibrium
Given frequencies of two alleles in a diploid population, the Hardy-Weinberg Principle may be used to predict the genotypes of offspring assuming random sexual reproduction, a large population, no mutations, and other conditions. If the observed genotypes of offspring match predictions, then it may be inferred that the gene frequencies are not changing (i.e. they’re in Hardy-Weinberg Equilibrium):
- Given two allele frequencies called p and q (i.e.
P(p)andP(q)), the sum of frequencies must be 1:p + q = 1 - Each gamete has probabilities p and q of having said alleles.
- Assuming random union of gametes into zygotes, the probability of two p alleles is p2, the probability of two q alleles is q2, and the probability of one p and one q allele is 2pq (from
(p + q)2). - The sum of frequencies of the offspring must be 1:
p2 + 2pq + q2 = 1
Protein Synthesis
- At a sequence of nucleotides called the Promoter, RNA Polymerase attaches and splits double-stranded DNA to perform transcription of genes into proteins. One promoter may transcribe one or more genes. In eukaryotic cells, certain proteins must first bind to a part of the Promotor called the TATA Box before RNA polymerase may bind.
- Transcription: RNA polymerase goes from the 3’ to the 5’ end of one DNA strand and adds to the 3’ end of a new RNA molecule called pre-messenger RNA (pre-mRNA, or the primary transcript, or heterogeneous nuclear RNA, or hnRNA). The name of the DNA strand is the template strand, or antisense strand, or negative strand. Since the pre-mRNA nucleotides are complementary to the template strand, they effectively represent the complementary DNA strand which is called the coding strand, or sense strand, or positive strand, or non-template strand. This is because the “sense” of the new pre-mRNA molecule reflects the sequence of bases in the sense or coding strand. By convention, a gene is described by its coding strand. Transcription proceeds at about 50 nucleotides per second. Transcription ends at a terminator.
- In eukaryotes:
- An enzyme adds a Guanine nucleotide to the 5’ end of the pre-mRNA, bound through a triphosphate, called the 5’ cap. An enzyme adds 50-250 additional adenine nucleotides to the 3’ end of the pre-mRNA, called the polyadenylation signal sequence (AAUAAA) or poly-A tail.
- The pre-mRNA is made of sets of nucleotides called exons (to be expressed) which are needed for translation, and introns (intervening sequences) which are not. A complex called a spliceosome made out of proteins and snRNA ribozymes cuts out the introns and connects the exons together, creating the final mRNA. Untranslated regions (UTRs) after the 5’ cap and before the poly-A tail are also left in the mRNA. A single gene may code for multiple proteins because of Alternative RNA Splicing depending on which segments are treated as exons.
- Translation: mRNA arrives at a free ribosome in the cytosol. A set of three nucleotides defines a codon which translates to an amino acid. Translation begins at an AUG codon (Methionine). As the ribosome moves over the mRNA molecule in the 5’ to 3’ direction, a transfer RNA (tRNA) with a matching codon (called an anticodon) attaches its matching amino acid to the new polypeptide chain at the Carboxyl end using rRNA. Translation stops at a stop codon UAG, UAA, or UGA using a release factor.
- Aminoacyl-tRNA Synthetases use ATP to create a tRNA and amino acid pair.
- A tRNA exits the ribosome at the E-site.
- A tRNA bound to the ribosome at the P-site adds its amino acid to the new polypeptide chain and moves to the E-site.
- A tRNA and amino acid pair for the next codon binds to the ribosome at the A-site (A for aminoacyl) and moves to the P-site when the other tRNA moves from the P-site to the E-site.
- If multiple ribosomes use a single mRNA in sequence, this complex is called a polyribosome (or polysome).
Gene Expression
Gene Expression describes when and why genes are used to create gene products such as proteins.
An operator is a part of a promoter (or in some cases, right after the promotor) which, if bound to by a repressor protein, inactivates the promoter and transcription of its gene(s). A molecule called a corepressor binds to the repressor to activate it to bind to the operator. The complex containing the promoter, operator, and gene(s) is called the operon. The genes in an operon are expressed together.
A repressible operon is usually on, unless turned off by a repressor. An inducible operon is usually off (because the repressor binds easily to the operator without a corepressor), unless turned on by an inducer which deactivates the repressor.
An activator is a protein that binds to the promoter to stimulate gene transcription by increasing the affinity of RNA Polymerase to the promoter.
All cells in an organism have the same genome (mostly, although some cells may have mutations), but only certain genes are expressed in different types of cells in a process called differential gene expression. Histones in a nucleosome have amino acid tails to which various functional groups may be attached.
When histone tails are unacetylated or methylated, the nucleosomes stay compacted and are unavailable for expression. When histone tails are acetylated, the nucleosomes become uncompacted and are available for expression.
In DNA Methylation, when certain bases are methylated on the DNA itself, expression may be inhibited. The methylation of genes may be replicated in mitosis or meiosis.
A constitutive gene is transcribed continually. A facultative gene is only transcribed when needed.
A transcription factor is a protein that controls the rate of gene transcription through activators and/or repressors. In eukaryotes, transcription factors may bind to one or more control elements (such as enhancers) of a gene which are non-coding DNA sequences that may exist upstream of the promotor and impact activity on the promoter through mediator proteins.
DNA may also transcribe non-protein-coding RNA called noncoding RNA (ncRNA) including microRNA (miRNA) and small interfering RNA (siRNA) which may inhibit mRNA translation. Another type of ncRNA called long noncoding RNA (lncRNA) has functions such as deactivating the second X chromosome in females.
Mutations
Changes to the genetic code of a cell are called mutations. If a mutation occurs due to physical or chemical means other than due to errors in DNA replication, such as a pyrimidine dimer, the agent is called a mutagen.
A point mutation is a mutation of a single nucleotide pair at a particular locus. Types of mutations:
- A nucleotide-pair substitution is a point mutation where one nucleotide pair is changed for another.
- A frameshift mutation occurs when a number of nucleotide pairs that’s not a multiple of three are inserted or deleted.
A single nucleotide base-pair where variation is found at a particular locus in at least 1% of a population of similar organisms is called a Single Nucleotide Polymorphism (SNP).
A silent mutation occurs when the new codon codes for the same amino acid. A missense mutation mutation occurs when the new codon codes for a different amino acid. A nonsense mutation occurs when the new codon is a stop codon.
DNA Self-Modification
DNA may modify itself (e.g. cut-and-paste or copy-and-paste) through transposable elements: retrotransposons and DNA transposons.
Retrotransposons are DNA sequences that use reverse transcription to copy-and-paste themselves in DNA. Reverse Transcription is the use of Reverse Transcriptase to create double-stranded DNA from RNA.
DNA Transposons are DNA sequences that use Transposase to cut-and-paste themselves in DNA.
Other DNA Behaviors
Restriction enzymes (or restriction endonucleases) detect particular sequences of DNA and cut the DNA into pieces at any such sequences (called restriction sites).
Epigenetics
Epigenetics is the study of heritable phenotype changes with causes other than changes to the DNA sequence such as methylation of histones on chromatin.
In some cases called genomic imprinting, there may be a difference in gene expression of a heterozygous allele on an autosomal chromosome depending on whether it came from the paternal or maternal chromosome (even though the DNA sequence would be the same in both cases).
Gene Therapy
Gene Therapy is the insertion of genes into somatic cells to change their function.
Transgenes are similar to gene therapy but introduced into zygotes and the resulting organisms are called Genetically Modified Organisms (GMOs).
Evolution
Evolution is the change of gene frequencies in a population over time. These changes may occur due to gene mutation, sexual reproduction, gene duplication, etc.
The theory of natural selection is that organisms that have characteristics best adapted for an environment (where the environment includes predators, selection by mates, artifical selection by other species such as humans, etc.) are more likely to gather resources, survive, and reproduce (quantified as Evolutionary Fitness), thus passing on their genotypes to offspring. These valuable traits are called adaptations.
Genetic Drift is evolution of certain alleles over others due to random chance (the smaller the population, the more likely). One type of genetic drift is the Founder Effect when a small part of a population becomes isolated from the larger population (e.g. on an island - this often also leads to the creation of multiple species, called allopatric speciation as opposed to sympatric speciation in the same geographical area). Another type of genetic drift is the Bottleneck Effect where a population has a significant decline due to some bottleneck such as a fire.
Gene Flow occurs when a new allele is introduced into a population from another population.
Homology (or homologous structures) is evidence of the existence of shared ancestry between a pair of structures, or genes, in different taxa due to descent with modification from a common ancestor. Convergent evolution is the independent evolution of similar traits and the traits are called analogous rather than homologous.
Stabilizing selection eliminates phenotypes at the extremes of a population characteristic. Directional selection favors one extreme of a population characteristic. Disruptive selection (or diversifying selection) favors both extremes of a population characteristic while selecting against the average.
Sexual Selection is a type of natural selection where individuals of one sex with certain characteristics are more likely to obtain mates than other individuals of the same sex. In Intrasexual Selection, organisms of the same sex compete for mates of the opposite sex. In Intersexual Selection, one sex (usually females) chooses the members of the opposite sex to mate with. Sexual selection may lead to Sexual Dimorphism which describes differences in characteristics between males and females.
In Frequency-Dependent Selection, the frequency of an allele fluctuates depending on the frequency of other alleles within the same species or in other species.
In Heterozygote Advantage, organisms with two or more alleles at a locus are selected at a higher rate.
A Hybrid Zone is a geographical area in which multiple species are more likely to reproduce (creating hybrids). Reinforcement is the process of increasing reproductive barriers between species in the case that hybrids are less fit than parents.
Allopolyploidy occurs when gametes of two different diploid species overcome sexual barriers and form a haploid zygote with non-homologus chromosomes. This zygote may develop into a mature organism through mitosis although it cannot reproduce sexually because meiosis requires paired chromosomes. However, nondisjunctions during mitosis may create a duplicated cell which has homologous chromosomes which will allow meiosis and subsequent sexual reproduction.
Proteomics
Proteomics is the study of proteins. The entire set of proteins expressed by a genome is called a proteome.
Prokaryotes
Prokaryotes are defined primarily by a lack of a membrane surrounding their DNA which instead is floating in an area of the cytoplasm called the nucleoid. Prokaryotes have an inner phospholipid bilayer cell membrane and most have a cell wall outside of the cell membrane to provide structure and protect from osmotic forces. In general, prokaryotes are about 1 micron in diameter.
Some prokaryotes may have an outermost, sticky capsule (or glycocalyx) of firmly attached polysaccharides and/or polypeptides extruded from the cell which protects against dessication. If the glycocalyx is loosely attached and water-soluble, it is called a slime layer instead of a capsule and may help attach to surfaces.
Prokaryotes have one or more circular DNA chromosomes (e.g. Vibrio has two, although with some debate about multiple bacterial chromosomes), and zero or more plasmids.
Most prokaryotes are capable of taxis which is movement toward or away a stimulus. Chemotaxis is taxis with a chemical stimulus.
Some prokaryotes may have flagella used for locomotion and sensing.
Some prokaryotes may have fimbriae which allow for sticking to surroundings instead of movement and are shorter than flagella.
Some prokaryotes may have pili (or sex pili; singular pilus) which are appendages that pull substances towards a cell (e.g. another cell for DNA transfer) or move by pulling on a stationary substance.
Prokaryotes asexually reproduce through binary fission (similar to mitosis although there’s no mitotic spindle). Binary fission may be initiated when DNA begins to replicate at an origin of replication.
Prokaryotic shapes are characterized as either coccus (spherical; plural cocci), bacillus (rod-shaped; plural bacilli), spiral (stiff spirilla or flexible spirochetes), or pleiomorphic (star-shaped, triangular, square, etc.). Curved bacilli with one curve are called vibrio. Curved bacilli with a few curves are called spirillum. Curved bacilli with more curves are called spirochaete.
If cells remain attached after binary fission, if there are two, the prefix diplo- is used (e.g. diplococci), if there are more than two, the prefix strepto- is used (e.g. streptococci). If fission and connection occurs in two planes, the groups are called tetrads. If fission and connection occurs in three planes, the groups are called sarcinae. If fission and connection occurs in random planes, the prefix staph- is used (e.g. staphylococci).
Prokaryotes are either classified as bacteria or archaea depending on differences in their rRNA.
Bacteria
Bacteria are prokaryotes with an an inner phospholipid bilayer cell membrane, most have a peptidoglycan cell wall (except for Mycoplasmas) made of polysaccharides on the outside of the inner membrane, a possible outer phospholipid bilayer membrane of lipopolysaccharides (LPS) and lipoproteins outside of the cell wall, and a possible further outer capsule or slime layer.
A Gram Stain may be used to differentiate bacteria based on their cell wall thickness. Gram-positive bacteria have a thick layer of peptidoglycan and stain as dark violet. Gram-negative bacteria have a thin layer of peptidoglycan and an additional outer layer of lipopolysaccharides and stain as pink or red. Both Gram-positive and Gram-negative bacteria may have a capsule. The outer phospholipid bilayer membrane is only found in Gram-negative bacteria.
Bacterial Flagella use a motor, hook, and filament.
Some bacteria may form endospores when there is a lack of nutrients which are a copy of its genome surrounded by keratin and peptidoglycan and the endospore pauses metabolism until nutrients are present again.
One bacterium may transfer some genes to another bacterium through various types of horizontal gene transfer:
- Transformation occurs when one bacterium dies and pieces of DNA may be released for uptake by other living bacteria in a state which is ready to take up new genes (this can be simulated by shocking bacteria with certain chemicals, electrical pulses, or heat shock followed by an ice bath).
- Conjugation is the transfer of genes between a bacterium with F-Factor (or fertility factor) which is a part of the donating cell genome and another bacterium through direct contact through a mating bridge. The donor cell is called the F+ Cell if the genes are in a plasmid and the F-Factor genes are transferred to the target cell (or F- cell) or the donor cell is called an Hfr Cell (High Frequency of Recombination) if the F-Factor is part of the main chromosome and the F-Factor is used to perform the conjugation but the actual transfer of genes is from another part of the chromosome that tags along with the F-Factor to the F- cell.
- Transduction occurs when a virus infects a bacterium and when copies of that phage end up in another bacterium, they may take with them some of the original bacterium’s DNA.
- Transposition is the movement of DNA segments (transposons) between chromosomes and plasmids across cells.
Quorum sensing occurs when one bacterium releases a signal molecule to communicate with other bacteria to detect and respond to bacteria population density.
Cyanobacteria are bacteria that obtain energy through photosynthesis.
Some groups of bacteria organize in groups called biofilms which are loosely bound by capsules and fimbriae and may coordinate metabolism. For example, Anabaena is a cyanobacteria which produces O2 as a product of photosynthesis, but this inactivates nitrogen fixation, so specialized cells called Heterocysts perform nitrogen fixation in biofilms next to the photosynthetic cells.
An average E. coli cell has about 35 billion atoms.
Antibiotics are substances which kill bacteria:
- Penicillin (and others in the β-Lactam family of antibiotics) acts as an irreversible competitive inhibitor on DD-transpeptidase which disallows it from building peptidoglycan cell walls which inhibits binary fission and causes bacterial death because natural cell wall destruction is not balanced with new cross-linking.
- Streptomycin kills the bacteria that cause Tuberculosis by binding to bacterial ribosomes (but not host eukaryotic ribosomes) and interfering with protein synthesis.
Some bacteria have resistance to some antibiotics with R Plasmids (resistance plasmids) that code for genes to resist such antibiotics.
Archaea
Archaea are prokaryotes without a peptidoglycan cell wall and have genetic similarities to eukaryotes which distinguishes them from bacteria.
Archaea include extremophiles that exist in conditions which hurt most other organisms. Extreme Halophiles live in high salt environments. Extreme Thermophiles live in high temperature environments.
Methanogens are archaea that release methane as a by-product.
Eukaryotes
Eukaryotes are single or multi-cellular organisms that have a nucleus containing chromosomes. Some have an additional cell wall like prokaryotes. In general, eukaryotes are approximately 10-100 microns in diameter.
Eukaryotic cells include organelles floating in the cytoplasm which provide various functions and are themselves contained in phospholipid bilayers. All of the organelles together are called the endomembrane system.
Protozoa is an informal, paraphyletic grouping of single-celled, heterotrophic eukaryotes that move and do not have a cell wall (therefore, are not plants nor fungi). Some protozoa have an outer envelope called a pellicle which is similar to a bacterial cell wall.
Amoeba is an informal grouping of single-celled eukaryotes that move using pseudopodia and may or may not have a cell wall.
Cell Nucleus
The cell nucleus is the largest organelle and contains the DNA. The cell nucleus is surrounded by the nuclear envelope which has a double membrane and nuclear pores for communication with the rest of the cell.
The nuclear lamina is an array of protein filaments that maintain the shape of the nucleus.
The nucleolus within the nucleus is made of fibers and DNA and where Ribosomes and rRNA are made.
In linear chromosomes such as those in Eukaryotes, the RNA Primer on the last Okazaki Fragment of the lagging strand cannot be replaced with DNA because there’s no 3’ end of another Okazaki Fragment to which DNA Polymerase can add nucleotides. Due to this, Eukaryotic have repetitive, non-protein coding nucleotide sequences of TTAGGG on each end of each chromosome called telomeres which are shortened during each replication. Telomerase may be used to extend telomeres such as for germ cells.
Eukaryotic Flagella
Eukaryotic flagella are anchored into the membrane with a basal body made out of microtubules.
Endoplasmic Reticulum
The endoplasmic reticulum (ER) is a network of membrane-enclosed spaces next to the nucleus. The area inside the membrane is called the lumen. The rough endoplasmic reticulum has ribosomes on the outside of its membrane and is involved in some protein synthesis. The smooth endoplasmic reticulum does not have ribosomes on the outside of its membrane and is involved in lipid synthesis, carbohydrate metabolism, and detoxification.
If a protein is planned to be secreted through the cell membrane, a signal recognition particle (or SRP), which is a protein-RNA complex, binds to a short chain of amino acids at the N-terminus of the newly synthesized protein called the signal peptide as it is being generated by a free ribosome. The SRP binding pauses translation and moves the polypeptide, SRP, ribosome and mRNA complex to the rough endoplasmic reticulum to finish translation inside the rough ER lumen.
Golgi Apparatus
The golgi apparatus is a stack of membrane-enclosed sacs called cisternae. Stacks closer to the ER are called the cis cisternae, and stacks closer to the cell membrane are called trans cisternae. When the ER synthesizes a protein, it’s placed in a transport vesicle which exits from the outside of the ER. These vesicles pass to and fuse with the cis cisternae. The cis cisternae then modifies and repackages the proteins and passes them on through the next layers of the golgi apparatus. Once the protein reaches the trans cisternae, proteins are sorted for their final destination.
Lysosomes
Lysosomes use hydrolytic enzymes to break down proteins, carbohydrates, and nucleic acids. Lysosomes have an acidic pH of 5.
Material may be first sorted inside endosomes before being processed by the lysosomes.
Peroxisomes
Peroxisomes use oxidative enzymes that catalyze reactions in which Hydrogen Peroxide is produced or degraded (e.g. removing Hydrogen in some metabolic pathways). In some organisms, glyoxysomes help break fats down into molecules for fuel.
Vacuoles
Vacuoles are membrane-enclosed sacs used to store waste products and help maintain structure.
Anatomy
Anatomy is the study of the structure of organisms. Physiology is the study of biological function, often a consequence of anatomy.
A tissue is the smallest collections of similar cells that carry out a particular function, along with an extracellular matrix that provides support for the tissue. The extracellular matrix is created by fibroblast cells. Integrin proteins on cell membranes bind to glycoproteins such as fibronectin in the extracellular matrix.
An organ is a collection of tissues with similar function.
A tract is a series of connected organs.
A lobe is a clearly visible division or extension of an organ.
Keratin is a strong protein that creates various structures in organisms.
Morphogenesis
Morphogenesis is the process that causes an organism to develop its shape such as the organized spatial distribution of cells during embryonic development.
A homeobox is a DNA sequence found within genes that encodes transcription factors related to morphogenesis. Homeosis is the transformation of one organ into another from mutation or misexpression of homeotic genes (genes with a homeobox).
Heterochrony is an evolutionary change in the rate or timing of various events in an organism’s development.
Paedomorphosis is the retaining of certain features of a child into a sexually mature age.
Energy Generation
Mitochondria and chloroplasts are organelles that generate the majority of usable energy in eukaryotes. The endosymbiotic theory (or symbiogenesis) is the idea that prokaryotic cells that resembled mitochondria and chloroplasts fused with protoeukaryotes to provide energy and received nutrients and protection in return. Both mitochondria and chloroplasts have their own DNA plasmids and ribosomes, although most of their proteins are created by the enclosing cell and imported.
Mitochondria
Mitochondria are the site of aerobic respiration. Mitochondria have an outer and inner phospholipid bilayer membrane with the space in between called the intermembrane space. The area inside the inner membrane is called the mitochondrial matrix. The inner membrane is heavily folded to increase surface area into structures called cristae.
Mitochondria have their own DNA called mtDNA which is passed down from the mother in the case of sexual reproduction. Mitochondria reproduce through binary fission like bacteria.
Hormones
Hormones are an intercellular communication mechanism over long distances and made of chemical signals that are part of the endocrine system which synthesizes and circulates such hormones, or simply primary messengers in cases where there is no endocrine system (such as in plants). Hormones are proteins, steroids, or amines. Amine Hormones are made from a single amino acid. Protein Hormones and most Amine Hormones are hydrophilic. Steroids and some Amine Hormones are hydrophobic.
Steroid hormones diffuse across the lipid membrane and bind to receptors in the cytosol or nucleus.
Tropic Hormones are hormones created in one endocrine organ but their target is another endocrine organ rather than a total body effect.
Paracrine Signals
Paracrine Signals are intercellular communication over shorter distances than hormones.
Autocrine Signals
Autocrine Signals are secreted molecules which trigger a response on the cells that secrete them.
Prostaglandins are either paracrine or autocrine signals which promote inflammation and the sensation of pain in response to injury.
Epithelial Tissue
Epithelial tissue lines the surface of organs, some inner cavities in organs, and the outside of the body. The main type of cell in epithelium is the epithelial cell.
In some organisms, the outside of the body forms a tissue called Skin which is made of the outermost epidermis, the inner dermis, and the innermost subcutaneous tissue (or hypodermis). In some organisms, the skin is part of the integumentary system which also includes hair and nails.
Transport Epithelia are layers of cells that move solutes in different directions to help with osmoregulation.
Some epithelial cells have fatty or lactic acids that inhibit bacterial growth, or secrete antimicrobial peptides that perturb membranes of invaders. Some epithelial tissues have cells that secrete mucus which is a thick fluid layer that contains glycoproteins, proteoglycans, and enzymes that protect the epithelial cells.
Connective Tissue
Connective tissues (or fibrous tissues) are found between other tissue types and provide various functions such as structural support. Connective tissues are made of fibers, a gel-like substance called ground substance, and various cells. The fibers include elastic fibers and collagen. Elastic fibers are bundles of Elastin and Microfibril proteins. Collagen is a structural protein embedded in a network of proteoglycans made of proteins and carbohydrates.
Bone is a strong and rigid mineralized connective tissue made by bone-forming cells called osteoblasts which deposit calcium, magnesium, and phosphate ions into a solidified matrix. Ligaments connect bones at joints.
Cartilage is a strong yet flexible material that contains fibers made of collagen embedded in a carbohydrate-protein complex called chondroitin sulfate. Cartilage is excreted by cells called chondrocytes.
Immune System
The immune system in an organism provides a defense system against pathogens (or germs) which are organisms that may cause various types of damage and/or disease, including bacteria, viruses, fungi, and parasites (a heterogenous classification of unicellular protozoa and multicellular invertebrates such as worms).
A disease (or sickness, or disorder, or morbidity, or pathology, or illness) is a condition that negatively affects normal structure or function of some part(s) of a host and may be caused by a pathogen or an internal dysfunction (such as an autoimmune disease where the immune system attacks its own host). An immunodeficiency disease is a genetic condition which introduces a defect in the immune system. An idiopathic disease has an unknown cause. An iatrogenic disease is one caused by someone trying to heal. A syndrome is a collection of diseases.
An endemic disease is one that has a generally consistent prevalence in a population. An epidemic disease spreads and increases significantly within two weeks. A pandemic disease is an epidemic disease that spreads quickly across a very large area.
A sign of a disease is an objective measurement or observation of something potentially related to a disease, whereas a symptom of a disease is generally considered a subjective description by the person with the potential disease.
An opportunistic pathogen is a mutualistic or commensal organism under normal conditions but may become parasitic if the host is weakened or if the organism moves into another area of the host where it may do damage.
Pathogens may inflict:
- Exotoxins: Proteins secreted by pathogens which cause damage. Hemolytic toxins (or hemolysins, or cytolytic toxins, or cytolysins, or cytotoxins) create holes in eukaryotic cells which causes water inflow and cell lysis.
- Endotoxins: Lipopolysaccharides of gram-negative bacteria released when the bacteria die and cause damage.
Non-pathogenic damage may be caused by:
- Poisons are chemical pathogens that may cause damage. Examples: Cyanide which interferes with the electron transport chain by binding to cytochromes.
Phagocytes are any cells that capture, engulf, and process dead or pathogenic cells or substances through the process of phagocytosis. The engulfed material becomes part of a phagosome inside the phagocyte which then fuses with a lysosome to create a phagolysosome which then digest the substances.
Some organisms use an enzyme called Lysozyme to break down pathogenic bacterial cell walls.
Animals
Animals are a kingdom of multicellular, eukaryotic, aerobic ([mostly][Yahalomi et al., 2020]), heterotrophic organisms that reproduce sexually, do not have cell walls (as in plants and fungi), and have specialized cells.
Some animals are bilaterians which have a two-sided symmetry and a digestive tract. Bilaterians have a dorsal top side, ventral bottom side, anterior front side, and posterior back side. Other animals have radial symmetry.
Cephalization is when an animal has a head, usually with a brain.
Key species that represent known evolutionary advances are:
- The ancestors of Sponges (Porifera) are thought to be the first animals introducing multicellularity, sexual reproduction (atlhough as hermaphrodites), and cellular communication. Sponges are suspension feeders (or filter feeders) eating organisms as they pass by.
- The ancestors of Jellyfish and Sea Anemones (Cnidaria) are thought to be the first animals with muscles, were motile (meaning they can move), had the first true tissues and organs, were the first active predators, and had the first nervous system.
- The ancestors of Flatworms (Platyhelminthes) are thought to be the first animals to have bilateral symmetry, a head, a complex brain, organs like kidneys, forward directed motion, and the first animals to be triploblastic (a third cellular layer in during development).
- The ancestors of Snails, Clams, and Octopus (Mollusca) are thought to be the first animals to have a shell, an adaptable body plan, a circulatory system, dynamic camoflauge, and water jet propulsion.
- The ancestors of Segmented Roundworms (Annelida) are thought to be the first animals to have segmentation (repetitive body segments), parapodia (fleshy protrusions), and a cuticle (non-mineral outer covering).
- The ancestors of Crustaceans, Barnacles, and Sea Spiders (Arthropoda) are thought to be the first animals to live on land, fly, have striated muscles, have joints, and have hemolymph.
- The ancestors of Starfish, Urchins, and Sea Cucumbers (Echinodermata) are thought to be the first animals to have an endoskeleton, protective spiny skin, and radial symmetry.
- The ancestors of Tunicates (Chordata) are thought to be the first animals to have a notochord, dorsal nerve cord, post-anal tail, and pharyngeal gill splits.
Animals may be coelomates when they have fluid- or air-filled cavities between the outer layer of the digestive tract and the outer skin. Acoelomates have coelems formed by mesoderm but it is fully filled with tissue.
Animals are split into vertebrates (with backbone) and invertebrates (without backbone and with an exoskeleton).
Herbivores are animals that eat mostly plants or algae. Carnivores are animals that eat mostly other animals. Omnivores are animals that eat plants, algae, and/or other animals.
Blood
Some organisms have Blood which is a liquid (and connective tissue) that includes blood cells, blood plasma, glucose, proteins, clotting factors, electrolytes, hormones, O2, CO2, hormones, waste, and more. Blood plasma is everything in the extracellular matrix of blood cells. Blood serum is everything in blood except for blood cells and clotting factors. Normal blood pH is about 7.4.
Blood Vessels
Blood vessels are either arteries, capillaries, or veins. The inner walls of blood vessels are lined with Endothelial Cells.
Arteries carry blood away from the heart to organs. Within organs, arteries branch into smell vessels called arterioles. Arterioles pass blood to capillaries which are very small, porous blood vessels that perform the exchange of gases and other chemicals between blood and cells. The ends of capillaries converge into venules which converge into veins that carry blood back to the heart.
A blood vessel contains a lumen surrounded by a single layer of endothelial cells. Blood pressure in vessels may be regulated by changing the diameter of vessels. Vasoconstriction increases blood pressure by reducing the diameter by constricting muscle cells around the vessel. Vasodilation decreases blood pressure by increasing the diameter of blood vessels (dilation) by relaxing muscle cells around the vessel.
Lymphatic System
All the fluid outside of cells is called extracellular fluid. The majority of extracellular fluid is called interstitial fluid, and the rest is blood plasma, and some other fluids.
The Lymphatic System is a part of the Circulatory System which has an alternative set of vessels called lymphatic vessels which transport a fluid called lymph, although lymph is not circulated by pumping of the heart but rather as a side effect of nearby moving muscles.
Lymph is interstitial fluid collected by the lymphatic vessels so that it may be transported to lymph nodes where it is cleaned and mixed back into the blood. Some of lymph is made of blood cells that diffused across blood vessel walls, but it is largely blood plasma.
Blood Cells
Blood cells (hematopoietic cells) are secreted in the bone marrow (before birth, in the yolk sac, liver, or spleen) from pluripotent hematopoietic stem cells in a process called hematopoiesis.
Hematopoietic stem cells may divide to create further hematopoietic stem cells in a process called stem cell self-renewal, or hematopoietic stem cells may specialize into one of three main cell types:
- Red Blood Cells (or erythrocytes, or RBCs) which lack a nucleus and most organelles and deliver O2 to cells in the body. They lack mitochondria and generate ATP through anaerobic respiration.
- Platelets (or thrombocytes) which are small packets of cytoplasm, lack a nucleus, and help with forming blood clots in response to injury. A blood clot that blocks the flow of blood in a vessel is called a thrombus. They lack mitochondria and generate ATP through anaerobic respiration.
- White Blood Cells (or leukocytes, or WBCs) which make up the immune system.
Innate Immune System
The immune system includes the innate immune system (or non-specific immune system) which has pre-defined defenses against particular types of pathogens.
The innate immune system detects pre-defined markers on the oustide of pathogens using soluble proteins and cell-surface receptors. Effector cells may attack marked pathogens directly.
The innate immune system also has pre-defined markers for viral infection on the inside of host cells. RIG-I-like receptors in cytoplasm detect viral nucleic acids (as nucleic acids should be inside the nucleus, not in the cytoplasm) and produce cytokines called Interferons. Interferons are released from the cell and act in an autocrine way on the same cell and in a paracrine way to alert nearby cells and leukocytes. This interferon response pathway interferes with viral genome replication and induces changes that make cells more likely to be phagocytosed.
Inflammatory Response
An inflammatory response (inflammation) is a cascade in response to injury or infection. Cells and proteins in the damaged area release cytokines (soluble proteins) that trigger the immune system. Cytokines induce capillary dilation which increases blood flow to the area which reddens and warms the area. Vascular dilation creates gaps in the blood vessel endothelium which leaks blood plasma into the connective tissue and this increase in fluid volume creates edema or swelling which puts pressure on nerves and causes pain (in addition to pain caused by other messengers). Cytokines also change the adhesive properties of the endothelium which increases the movement of white blood cells into the damaged area.
Adaptive Immune System
Some vertebrate animals include an adaptive immune system (or acquired immune system, or specific immune system) which is in addition to the innate immune system and uses specialized cells for more targeted defense against pathogens.
Immune System Specialization
The tree of cell types generated by hematopoietic stem cells is:
- Erythroid: This cell type may further specialize into:
- Erythroblasts: These cells create erythrocytes.
- Megakaryocytes: Cells with chromosomes from multiple nuclei arising from the fusion of multiple precursor cells. Megakaryocytes create platelets.
- Myeloid are leukocytes in the innate immune system which may further specialize into:
- Granulocytes (or polymorphonuclear leukyocytes): Cells containing reactive substances that kill microorganisms and increase inflammation. These cells may further specialize into:
- A Neutrophil cell which is a phagocytic leukocyte effector cell attracted by signals from infected tissues and can work in anaerobic conditions. Neutrophils die at the site of infection and are the main constituent of pus. Neutrophils are so named because their granules are neither acidic nor basic.
- An Eosinophil cell which is a leukocyte that targets multicellular invaders and defends against helminth worms and other parasites. These are so named because their granules contain basic substances that stain with acids such as eosin.
- A Basophil cell which is also related to parasites. These are so named because their granules contain acidic substances that stain with bases such as hematoxylin.
- A Mast cell resident in connective tissue which releases signaling molecules called histamines which stimulate surrounding blood vessels to dilate to increase blood supply and become more permeable to neutrophils.
- A precursor cell which may further specialize into:
- A Monocyte cell which circulates in the blood and may further specialize into:
- A Macrophage which is a mature monocyte that has left the blood and reside in tissues as a phagocytic, sedentary cell that kills pathogens or cleans up dead cells. Macrophages also release cytokines which attract neutrophils and other leukocytes to the area.
- A Dendritic cell which populates tissues such as skin and migrates to lymph nodes after capturing intact or degraded pathogens to stimulate the adaptive immune system.
- A Monocyte cell which circulates in the blood and may further specialize into:
- A Nuocyte cella which is also related to parasites.
- Granulocytes (or polymorphonuclear leukyocytes): Cells containing reactive substances that kill microorganisms and increase inflammation. These cells may further specialize into:
- Lymphocytes (Lymphoid) are leukyoctes found predominantly in lymph. This cell type may further specialize into:
- A B cell which is in the adaptive immune system and may further specialize into:
- A Plasma cell: An effector B cell.
- A precursor cell which may further specialize into:
- A Natural Killer cell is a large granulocyte effector cell in the innate immune system which circulates throughout the blood and lymph patrolling for virus-infected and cancerous cells. Instead of phagocytosis, natural killer cells release cytokines that cause cell death or inhibit viral replication.
- A Naive T cell which is in the adaptive immune system and may further specialize into:
- A Cytotoxic T cell which is a granulocyte effector T cell.
- A Helper T cell that helps activate other immune system cells such as B cells.
- A B cell which is in the adaptive immune system and may further specialize into:
Phagocytosis may be driven through Toll-like receptors (TLRs) in phagocytic membranes which bind to pathogens and bring them inside the cell into vacuoles through endocytosis:
- TLR1 recognizes lipopeptides on some bacteria.
- TLR2 recognizes glycosylphosphatidylinositol and lipoteichoic acid on some parasites and gram-positive bacteria.
- TLR3 recognizes double-stranded RNA produced by some viruses.
- TLR4 recognizes lipopolysaccharides on the surface of Gram- bacteria or Lipotechoic acid on Gram+ bacteria.
- TLR5 recognizes the flagellin protein on bacterial flagella.
- TLR6 recognizes zymosan on some fungi.
- TLR7 recognizes some single-stranded viral RNAs.
- TLR8 recognizes some single-stranded viral RNAs.
- TLR9 recognizes unmethylated CpG nucleotide motifs on some bacterial and viral genomes but not in human DNA.
The complement system of the innate immune system is made of proteases that circulate in blood as inactive zymogens. The Complement Component 3 (C3) molecule is made in the liver. The coating of a pathogen with markers such as C3b (a fragment of C3) is called opsonization and alllows phagocytes to process the pathogen using receptors such as Complement Receptor 1 (CR1), Complement Receptor 3 (CR3), and Complement Receptor 4 (CR4). There are three known ways of C3b activation: the classical pathway, the Lectin pathway, and the alternative pathway.
In the alternative pathway, as C3 circulates, it spontenously changes its conformation at a slow rate to expose a thioester bond on the inside of the protein to the aqueous blood which makes a covalent bond to a molecule with an amino or hydroxyl group such as water. The environment near certain pathogens such as bacteria increases the rate of this hydrolization. This hydrolized C3 binds to Factor B which makes this combined molecule susceptible to cleavage by Factor D. This reaction produces fragments Ba and Bb and Bb remains bound to C3 (C3Bb), together called a C3 Convertase, and the C3 Convertase cleaves C3 into a small, soluble C3a fragment and large C3b fragment. The C3b fragment covalently bonds to amino and hydroxyl groups on the pathogen. The pathogen-bound C3b can still further bind and cleave more Factor B which acts as more C3 Convertase to cleave other C3 near the pathogen and this positive-feedback loop may coat the pathogen in C3b. This behavior may be enhanced by Properdin (Factor P). Factor H and Factor I work to decrease C3 convertase effectiveness on the pathogen because otherwise the reservoir of C3 might be exhausted and too few C3 could attack all available pathogens. The Decay-Accelerating Factor (DAF) and Membrane Cofactor Protein (MCP or CD46) use a cell-surface carbohydrate binding site of sialic acid that most bacteria do not have (exceptions include Streptococcus pyogenes and Staphylococcus aureus) and act to avoid too much C3 binding to host cells.
C3b may also create a C5 convertase which cleaves C5 into small C5a and large C5b fragments. C5b interacts with C6, C7, C8, and C9 to create a membrane-attack complex which makes holes in cell membranes that cause cell lysis (known to be particularly effective strains of Neisseria that cause gonorrhea and one form of bacterial meningitis). As with Factor H, Factor I, DAF, and MCP, there are proteins that act to inhibit the membrane-attack complex on human cells: Protein S, Clusterin, Factor J, Homologous Restriction Factor (HRF), and Protectin (CD59). The disease paroxysmal nocturnal hemoglobinuria is the inability to link DAF, HRF, and CD59 to the plasme membrane with glycosyl phosphatidylinositols.
The C3a and C5a fragments acts as a chemoattractant to recruit effector cells such as phagocytes, and increase inflammation by stimulating smooth muscle contraction, and activate mast cells and basophils which release histamines, and act on neutrophils and monocytes to increase their adherence to cell walls and increases CR1 and CR3 expression. They are anaphylatoxins because they may cause anaphylactic shock which is an inflammatory reaction that occurs simulataneously in tissues throughout the body.
The classical pathway may be activated by the binding of C-reactive Protein to a pathogen (e.g. to lipotechoic acid on Gram-positive bacteria) which then interacts with C1 complex which leads to C4 and C2 cleavage that create different type of C3 convertase.
The Lectin pathway may be activated by Mannose-Binding Lectin which may bind to certain conformations of mannose on pathogens and may ultimately lead to a C4C2 C3 convertase.
Myeloid cells are effector cells that have cell-surface receptors for general classes of pathogens, rather B and T lymphoid cells which have specialized receptors for particular pathogens. B and T Lymphoid cell-surface receptors are not directly coded by specific genes but instead are created by genes that are cut and modified during development to generate millions of different receptors.
Antigens are molecules on pathogens or released by pathogens that cause a response by B or T cells. B or T cells (or B cell products) bind to an antigen with proteins called paratopes (or antigen receptors). A single B or T cell presents a single type of paratope. Antigens are made of one or more epitopes which are the parts of an antigen that bind to the corresponding paratopes.
B cells mature in the bone marrow and bind to antigens on non-host cells or which are free floating in blood. Each B cell has Y-shaped protein complexes made of four polypeptide chains linked with disulfide bridges, two of which called heavy chains are anchored into the B cell membrane. Each chain has a constant region which is an amino acid sequence that is common across B cells, and a variable region which is an amino acid sequence that varies for a particular epitope. The two pairs of chains may bind to up to two epitopes.
Once a B cell’s paratopes bind to an antigen, B cells become activated and lead to formation of effector cells called Plasma cells that secrete a soluble form of the paratope proteins called antibodies (or immunoglobulins) which are free floating in blood and lymph and bind to antigens and interfere with pathogens or their excreted toxins (neutralization), or mark pathogens for destruction (opsonization) by phagocytes, or mark pathogens for destruction by the complement system’s classical pathway by acting as an intermediate to binding the C1 Complex.
There are five types of B cell antibodies (with Ig an abbreviation for immunoglobulin): IgM, IgG, IgD, IgE, and IgA.
T cells mature in the lymphatic system organ called the thymus and bind to antigens on host cells called antigen-presenting cells (such as dendritic cells) that present antigens (only parts of proteins) connected to Major Histocompatibility Complex (MHC) molecules (class I MHC which presents intracellular pathogens or class II MHC whichs present extracellular pathogens). A T cell paratope is a protein made of two polypeptide chains (called the α chain and β chain) anchored into the T cell membrane and linked with a disulfide bridge. Each chain also has a constant region and variable region and the pair of chains are the paratope.
Cytotoxic T cells are like specialized Natural Killer Cells and defend against intracellular pathogens by detecting class I MHC-bound proteins on infected cells using a protein called CD8. After binding, the cytotoxic T cell secretes proteins such as Perforin and Granzymes that stimulate apoptosis of matching cells.
Helper T cells defend against extracellular pathogens by detecting class II MHC-bound proteins on antigen-presenting cells such as dendritic cells, macrophages, and B cells using a protein called CD4 (usually associated with Helper T Cells). This binding releases cytokines which activates the T cell and stimulates cloning and cytokines which stimulate cloning of B cells. Regulatory T Cells are Helper T cells that help control the activities of the other T cell types to limit potential damage.
B and T cell antigen receptors are encoded in immature lymphocyte DNA through V(D)J recombination by V (variable) and J (joining) peptide sequences which are combined in random pre-mRNA combinations by recombinase.
As B and T cells mature in the bone marrow or thymus, respectively, antigen receptors are tested for self-reactivity and destroyed through apoptosis if they would attack host cells.
Once B or T cells bind to antigens, they start to rapidly reproduce into a group of identical cells called a clone that can match such antigens. Clones are monoclonal if a single clone reacts to a single epitope on an antigen, and polyclonal if multiple clones react to different epitopes on the same antigen. This adaptive immune system process is called clonal selection and clonal expansion which is part of the primary immune response and peaks about a week or two after initial antigen recognition. A subset of the clone become effector cells which are immediately able to act against antigens. When an infection dies down, some of the remaining effector cells survive apoptosis and remain as memory cells in case the same infection returns.
B and T lymphocytes may move throughout both blood and lymph. Mature lymphocytes enter the blood and then drain into lymph and finally lymph nodes. If a lymphocyte is activated, it remains in the lymph node.
If an antigen matching memory cells is seen after initial exposure, the memory cells act as part of the stronger secondary immune response and typically peak a few days after infection.
In humans, the spleen is the only lymphoid organ that acts to filter pathogens in circulating blood (in addition to removing damaged erythryocytes).
Septic shock may occur when a bacterial infection (often Gram-negative) spreads to the blood which drives macrophages to release TNF-α which causes widespread dilation of blood vessels which leaks a lot of fluid into tissues and may cause blood pressure to drop so low that organs may fail.
A response to pathogens in the blood and lymph is called a humoral immune response. A response to pathogens which doesn’t involve antibodies is called a cell-mediated immune response which uses specialized T cells to destroy infected host cells.
Vaccines are injections of antigens (often without an activated pathogen) which stimulate the primary immune response and confer active immunity.
Passive immunity is the transfer (or injection, e.g. antiserums such as antivenoms and antitoxins) of antibodies which last temporarily.
Immune System Notes
The glycocalyx capsule or slime layer of some prokaryotes (e.g. Streptococcus pneumoniae, Klebsiella pneumoniae) have chemicals that are generally present in the host so they are not recognized as pathogens.
The the lipid portion of the LPS in the outer phospholipid bilayer membrane of Gram-negative prokaryotes is named Lipid A and this may trigger fever, vasodilation, inflammation, shock and blood clotting when it is released as part of cell death. Therefore, killing many Gram-negative prokaryotes at the same time may release too much Lipid A for the body to handle.
Fungi
Fungi (singular fungus) are a kingdom of uni- or multi-cellular eukaryotic organisms which are heterotrophs and have cell walls made of chitin. Fungi include yeasts, molds, and mushrooms. Fungi break down organic material and recycle nutrients. Yeasts are single-celled fungi. Some fungi such as some yeast have DNA plasmids.
Most fungi spend most of their life as haploid organisms with a brief diploid period after fertilization which quickly transitions via meiosis back to a haploid organism.
Multi-cellular fungi form a network of tiny filaments called hyphae which form tubular cell walls around the membranes of fungi cells. Hyphae are divided by walls called septa (singular septum) unless the fungi are coenocytic. Hyphae form an object called mycelium which invades the material the fungi is eating.
Many fungi reproduce both sexually and asexually. Two mycelia may merge into a plasmogamy which shares two nuclei called heterokaryon in a structure called a zygosporangium or combine more fully into a dikaryotic, multi-nuclei cell. In the process of karyogamy, the two haploid nuclei fuse into diploid cells. A fungal spore is called a conidiophore which forms in a sporangium called a sporangiophore.
A lichen is a grouping of many photosynthetic organisms within a fungal hyphae.
Infection of an organism by a parasitic fungus is called mycosis.
Fungi and plants may combine to form mycorrhizae in which fungi supply nutrients (e.g. Phosphorous), minerals, and some antibiotics to plants and plants supply sugar to the fungi. Branching hyphae called arbuscules allow the fungi to exchange nutrients with plant hosts.
Algae
Algae are aquatic, photosynthetic, eukaryotic, single or multi-cell protists which don’t have as complex a vascular system nor as complex a reproductive system nor as complex tissues as plants so they’re not considered plants. Most algae spend most of their life as haploid organisms with a brief diploid period after fertilization which quickly transitions via meiosis back to a haploid organism.
Plants
Plants are photosynthetic, eukaryotic, autotrophs and have terrestrial adaptations that algae lack such as complex tissues. Plants have a cell wall made of cellulose. Plants evolved from freshwater multicellular algae.
Plants have alternation of generations and they are embryophytes which use embroys to nurture young sporophytes within the gametophyte.
Plants are also called land plants (or terrestrial plants) because they live on or are closely related to land which is a solid surface of the Earth not covered in water. Plants usually require soil for survival.
Soil is a part of land that usually has nutrients, minerals, water, gases, and organic matter. Soil forms from the weathering of rock, the remains of dead organisms (called humus), and other organic material. Topsoil is the uppermost layer of soil that has the highest concentration of organic matter. The most fertile topsoils are called loams. Vegetation is a group of plants that cover soil.
Plant roots are organs that lie below soil and absorb water and minerals and anchor the plant into the soil. Taproots have a single root extension into the soil while others are branched. Some roots have root hairs to increase surface area to acquire resources. Others without root hairs have a symbiotic relationship with attached bacteria to increase surface area to acquire resources. Rooted plants help an area avoid erosion which is the removal of soil due to physical processes. Roots use cation exchange by acidifying the solution with H+ which displaces mineral cations in the soil which the roots then absorb. Phytoremediation is the use of certain plants (e.g. alpine pennycress) that extract harmful minerals for disposal.
Plant shoots are everything above ground. The plant stem lies above the soil (in general) and supports photosynthetic organs. Growth mostly occurs at the terminal tip called the apical bud. An axillary bud may create a lateral branch, thorn, or flower.
Branches are stem-like appendages that grow from the stem and hold leaves to the light and transport water and nutrients between the roots and leaves.
Plant leaves are organs that project from branches and perform photosynthesis. The lower epidermis of leaves includes stomata (singular stoma) which are openings that allow diffusion of Carbon Dioxide, Oxygen, and water vapor into and out of the leaf. The arrangement of leaves on a stem is called phyllotaxy.
Guard cells open stomata during the day to allow in Carbon Dioxide for photosynthesis and close at night to limit transpiration which is the process of water evaporation in plants. Water in a leaf exerts pressure called turgor pressure causing the swelling or clamping down of the stomata to regulate their size. The guard cell includes blue-light receptors which open Potassium ion channels when exposed to blue-light in the day and let in Potassium ions and thus water flows into the guard cells to follow its osmotic gradient.
Everything in cytosol in all the cells and the plasmodesmata is called the symplast. Everything that’s living in the cell, including the plasma membrane, is called the protoplast which is a superset of the symplast. Everything external to the cell membranes is called the apoplast.
Covering the epidermis, plants have a cuticle which is made of wax and other polymers to prevent excessive water loss.
Plant sporophytes have organs called sporangia that produce spores from diploid cells called sporocytes. Leaves that have sporangia are called sporophylls (groups of which may be called sori or strobili). Homosporous plants have one type of sporophyll that produces a bisexual gametophyte whereas heterosporous plants are split into those that produce female versus male gametophytes (megasporangia and microsporangia, respectively). Heterosporous seeded plants have tissues called integument that protects their megasporangium with the whole structure called the ovule. A microsporangium produces a microspore which develops into pollen that includes the gametophyte and an enclosure. The transfer of pollen to the ovule is called pollination.
Plant gametophytes have organs called gametangia (female archegonia and male antheridia) which produce gametes that fertilize in the archegonia.
Phototropism is the directional growth of plants towards light.
Vascular plants (tracheophytes) have vascular tissues (steles) which propagate resources to the entire plant, allowing for very large plants. These vascular tissues include xylem and thinner-walled phloem. In some cases, stele is a combination of xylem and phloem whereas in others, they are separate. Xylem and phloem transport liquid and dissolved substances (collectively called sap) using bulk flow from higher to lower pressure independent of solute concentration (as in osmosis). Vascular plants have a dominant sporophyte generation.
Transpiration creates negative pressure which drives additional water uptake and bulk flow of sap upwards (using hydrogen bonding to create water cohesion) through the vascular system. Areas with a lot of nutrients create an osmotic pressure for water to enter and thus allow for the “long-range” diffusion of nutrients.
The xylem transports most water and minerals in sap from the roots to the shoots using tracheid cells and vessel element cells which have walls strengthened with the polymer lignin. In a mature xylem, both cell types are dead even as they function. At night, when there is little transpiration, root cells continue to pump in ions into the xylem which lowers the water potential pressure, which leads to water pumping in due to osmosis, which generates root pressure to push sap up the xylem. If root pressure pushes sap faster than the rate of transpiration, this results in guttation which is the excretion of water droplets on plant leaves, although this should not be confused with dew which is condensed atmospheric moisture.
The Phloem has cells arranged in tubes that transport sugars, amino acids, and other organic products from the leaves to where they are needed or stored. Phloem sap can flow in both directions. This transportation is called Translocation. Sap travels through conduits in the phloem called Sieve-Tube Elements connected through plasmodesmata to Companion Cells (which provide the nucleus and ribosomes for the sieve tube elements) and supported by Bast Fibers.
The soil immediately surrounding roots is called the Rhizosphere and may contain rhizobacteria which are bacteria that live in the rhizosphere. Endophytes are rhizobacteria or fungi that live between plant cells. Nitrogen is an important macronutrient for plants taken up during the Nitrogen Cycle and it comes from Rhizobacteria which use Nitrogen Fixation to convert Nitrogen gas from the air (N2) into Ammonia (NH3) which combines with H+ in the soil to form Ammonium (NH4), or Nitrogen from humus directly into Ammonium. Ammonium may be taken up directly by plant roots, or it may be further processed by Nitrifying bacteria into Nitrite (NO2-) which is then processed by other nitrifying bacteria into Nitrate (NO3-) which is taken up by plant roots. Weathering of rock and lightning striking Nitrogen gas may also create Nitrate which is taken up by plant roots. Nitrate may by converted into Ammonium in the plant root, or sent up the xylem and converted into Ammonium near where it’s needed. Ammonium absorbed from soil is also taken up the xylem. Crop rotation usually involves periodically planting legumes with rhizobacteria called Rhizobium which are particularly good at increasing Nitrogen concentration in the soil.
Plants have undifferentiated tissues called meristems which contain stem cells that can divide and differentiate, allowing for indeterminate growth to increase the root and shoot ends of the plant through apical meristems (called primary growth). Any tissues that are neither vascular nor epidermal are called Ground Tissue (Parenchyma cells in soft tissues, Collenchyma cells for structure in new growth, and Sclerenchyma cells as the main structural support). Ground tissue internal to the vascular tissue is called Pith. Primary growth results in the following tissues: Protoderm (which develops into the epidermis), Procambium (which develops the vascular primary xylem and phloem tissues surrounded by the Pericycle which is surrounded by the Endodermis), and Ground Meristem (which develops into the Cortex between the endodermis and epidermis).
Woody plants also have growth in thickness (called secondary growth) through lateral meristems called the vascular cambium and cork cambium. The vascular cambium is the main lateral growth layer where vascular tissue is added called secondary xylem (or Wood) to the outside of the primary xylem, and secondary phloem to the inside of the primary phloem. Bark is all tissue external to the vascular cambium. The part of bark that replaces the epidermis with tougher periderm is called the cork cambrium which laterally grows Cork cells.
The Casparian Strip is a strip within endodermis cells in the root made of suberin - a waxy, hydrophobic substance - which blocks the entrance of water and minerals from the epidermis of root hairs through the apoplast; instead, they must detour through the symplast which only allows in certain substances.
Some vascular plants use seeds which are packages of nutrients, an embryo, and a protective covering. The first round of mitosis of a plant zygote creates the terminal cell which will create most of the embryo and the basal cell which creates a structure of cells called the suspensor which anchors to the endosperm and parent plant to exchange nutrients. The cotyledons (which will nourish the embryo) form from the proembryo created by the apical terminal cells. A seed embryo is attached to the Plumule which includes Cotyledons, the Hypocotyl (the embryonic axis) which terminates in the Radicle (or embryonic root), the Epicotyl (above the Hypocotyl), and in some plants, shoots are sheathed in Coleoptiles, and roots are sheathed in Coleorhizas. The process of absorbing water and breaking a period of dormancy of a seed is called germination which is started by imhibition that uptakes water because the seed is so dry. A seed is broken and starts germination depending on various conditions such as environmental cues and most seeds can last up to a few years. Seed-based plants have a dependent gametophyte generation in which the gametophyte is dependent for survival on the parent sporophyte.
Vascular plants that do not produce seeds are either lycophytes or monilophytes.
Angiosperms (or flowering plants) have flowering seeds whereas gymnosperms have naked seeds. Most angiosperms are hermaphrodites and some may self-pollinate. Gymnosperms that create cones are called conifers.
A flower on an angiosperm is made of sepals which enclose the flower before it opens, petals which are colored leaves that attract pollinators, stamens (made of a stalk called a filament) which produce the microsporangium (and thus pollen) in a sac called the anther at the end of the filament, carpels which produce the megasporandium (and thus ovule) with a stigma that receives pollen which leads to the style that leads to the ovary through the micropyle that contains the female gametophyte (a single carpel is a pistil). After fertilization into a sporophyte and cotyledon (initial root and leaves), the mature ovary develops into fruits (with seeds) which protect the seed and aid in its dispersal. In the process of double fertilization, another sperm produces an additional triploid cell which turns into endosperm that nourishes the embryo. A vegetable is any edible part of a plant which is not the fruit.
Nonvascular plants are called bryophytes including mosses, liverworts, and hornworts.
Microphylls are leaves with no vascular branching and megaphylls have high vascular branching.
Monocots are flowering plants that have one cotyledon in the embryo, and leaves that are narrow with veins that run parallel to the length of the leaf.
Eudicots are flowering plants that have two cotyledons in the embryo, and leaves that are broad with veins arranged in a net.
The ABC Model of Flower Development describes how three genes in meristems lead to the four types of flower organs (A genes in the outer sepals and petals, B genes in the middle petals and stamens, and C genes in the inner stamens and carpels). Sepals arise where only A genes are active. Petals arise where only A and B genes are active. Stamens arise where only B and C genes are active. Carpels arise where only C genes are active.
An epiphyte is a plant that grows on another plant, gathering resources independently. Parasitic plants are like epiphytes except they extract resources from the host plant.
Etiolation is the process of executing morphological changes in response to a lack of light. As light becomes available (e.g. to stems that break through the top layer of soil), the process of de-etiolation (or greening) executes different morphological changes to complete maturation.
Photoperiodism is the ability to act differently based on the length of a day (thus detecting the season of the year). Short-day plants flower when the length of night in a day is longer than a threshold. Long-day plants flower when the length of night in a day is shorter than a threshold. Day-neutral plants’ flowering is unaffected by the length of light in a day.
Some plants require certain temperature thresholds before flowering and this may be induced through a process called vernalization.
Some plants respond to the effects of gravity through the process of gravitropism which detects the settling of cytoplasmic components called statoliths.
Some plants respond to mechanical stimuli such as wind through the process of thigmotropism.
Chloroplasts
Mitochondria-like organelles in plants and algae are called Plastids and have their own DNA like mitochondria. Chloroplasts are plastids that act as the site of photosynthesis. In chloroplasts, the outer membrane is not used as part of ATP synthesis like in mitochondria but is a relic of endosymbiosis.
Within the inner membrane, in the fluid called the stroma, chloroplasts are further divided into membraned sacs called thylakoids which are arranged into stacks called grana (a single stack being a granum). Chlorophyll molecules in the thylakoid membranes absorb light and the Calvin Cycle uses that energy to synthesize organic compounds.
Plants have leaves which include mesophyll cells that contain chloroplasts (the mesophyll cells on the top of the leaves exposed to the most sunlight are Palisade Cells). Mesophyll cells have both chloroplasts and mitochondria. Between the mesophyll cells and the vascular system (e.g. phloem) are protective Bundle-Sheath Cells. Sugars from photosynthesis flow from mesophyll cells into bundle-sheath cells into phloem parenchyma, then into companion cells (using an active transport that needs ATP as part of Phloem Loading) and finally into the sieve-tube elements in the phloem. The Pressure Flow Hypothesis proposes that in some plants, loading sugars into phloem causes osmosis of water from nearby xylem thus creating a water pressure potential gradient and the rest of the phloem and the sugars are pressured into flowing towards destinations that have lower sugar concentrations.
An alternative and less common method of Carbon fixation is C4 Carbon fixation which creates 4-Carbon products (instead of 3-Carbon products) in the Calvin Cycle using PEP Carboxylase. C4 plants finish the Calvin cycle in the chloroplasts of bundle-sheath cells instead of mesophyll cells and concentrate more CO2 around RuBisCO to increase RuBisCO’s fixation efficiency. Although this process is more efficient, it also uses more energy.
Some plants in arid conditions are called xerophytes and use Crassulacean Acid Metabolism (or CAM) as opposed to C3 or C4 in which stomata open during the night and fix Carbon into vacuoles which are used during the day in the Calvin Cycle.
Plant Hormones
Under conditions of little water (e.g. drought), Abscisic Acid (ABA) is produced by roots and leaves to signal guard cells to close stomata to reduce transpiration.
Auxin stimulates stem elongation by stimulating cell wall proton pumps which lowers the pH inside the cell and the acidification activates Expansins which break down parts of the cell wall that inhibit growth, and additional ion uptake from the Auxin creates a hyperosmotic cell which brings in more water and the cell wall flexibility and increased turgor pressure elongates the cell.
Cytokinins (usually made of Adenine) stimulate cell division and differentiation in shoots and roots.
Gibberellins stimulate stem elongation, fruit growth and seed germination.
Ethylene promotes ripening of fruits and a response to stress to strengthen the stem and/or grow horizontally to avoid obstacles.
Brassinosteroids promote cell expansion and division in shoots.
Jasmonates regulate fruit ripening, floral development, and more.
Strigolactones promote seed germination.
Plant Immune System
Plants recognize Pathogen-Associated Molecular Patterns (PAMPs) which lead to a chain of defensive events such as the production of phytoalexins.
Effector-Triggered Immunity deals with pathogens that avoid detection by PAMPs (through the use of effectors that block PAMP perception) by defensive genes that are stimulated by the effectors. Effector-triggered immunity initiates the hypersensitive response which leads to localized cell death near the infection site. Systemic Acquired Resistance occurs as a plant-wide expression of defensive genes through signaling molecules such as Salicylic Acid.
Cell Communication
Tight junctions connect the outside of two cells together using various proteins to create a seal.
Desmosomes connect the outside of two cells together using intermediate filaments made of keratin.
Gap junctions directly connect cytoplasms of two cells to allow exchange of some substances.
In plants, Plasmodesmata directly connect cytoplasms of two cells in a way like gap junctions, but allowing even more through. Actin proteins contribute to a circular flow or cytoplasm called cytoplasmic streaming. The remaining area between plant cells is called the middle lamella made of polysaccharides called pectins.
Signaling Pathways
A signaling pathway (or signaling cascade, or biochemical cascade) is a series of chemical reactions initiated by an external stimulus (or first messenger) such as a non-steroid hormone or other ligand which cannot cross the cell membrane but binds to a membrane receptor. The first messenger bound to the membrane receptor signals second mesengers which drive the process of signal transduction that triggers effectors to take action inside the cell. Cytokines are proteins that are first messengers.
Common transmembrane receptors:
- G Protein-Coupled Receptors (GPCRs) couple with a signaling molecule that binds to the GPCR on the outside of the cell, and a G protein in the cytoplasm (activated when a GDP is replaced by GTP), after which the G protein and GTP complex unbinds from the GPCR and binds to a transmembrane enzyme such as Adenylyl Cyclase to activate it and signal a second messenger.
- Receptor Tyrosine Kinases (RTKs) couple with a signaling molecule that binds to the RTK on the outside of the cell which leads to coupling with neighboring RTKs which activates tyrosine kinases in the cytoplasm (with ATP) that signal second messengers that bind to the phosphorylated receptors.
A major difference between GPCRs and RTKs is that GPCRs generally activate a single signal pathway whereas RTKs may trigger many signal pathways.
Second messengers are either proteins or:
- Cyclic AMP (or cAMP) which activates the effector Protein Kinase A (PKA). Adenylyl Cyclase transmits a signal by converting ATP to cAMP, often activated by a G Protein.
- 1,2-diacylglycerol (or DAG) activates the effector Protein Kinase C (PKC).
- Inositol trisphosphate (or inositol 1,4,5-trisphosphate or IP3) which can travel through the cytoplasm to the endoplasmic reticulum to stimulate the release of Ca2+ inside the cytosol.
- Ca2+ ions which can cause muscle contraction.
Scaffolding proteins bind to a transmembrane receptor and multiple relay proteins together to increase the speed of signal transduction.
Circulatory System
The Circulatory System (or cardiovascular system) transports nutrients, Oxygen, CO2, hormones, waste and more throughout an organism using blood (in a closed circulatory system) or hemolymph (in an open circulatory system) through tubes called vessels.
Heart
The heart is an organ that pumps blood through the circulatory system. The combination of the heart and blood vessels is called the Cardiovascular System.
Hearts contain two or more chambers. Chambers that receive blood in are called atria and chambers that pump blood out are called ventricles. Systemic Circulation carries oxygenated blood from the heart to the body. Pulmonary Circulation carries deoxygenated blood from the heart to the lungs. The major steps of heart function are:
- Contraction of the right ventricle pumps blood to the lungs via Pulmonary Arteries where blood loads O2 and unloads CO2.
- Oxygenated blood returns from the lungs via the Pulmonary Veins into the left atrium and then the left ventricle and then out through the artery called the aorta.
- After the arteries and veins weave through the body, de-oxygenated blood from the head, neck and forelimbs flows into a vein called the Superior Vena Cava which feeds back into the right atrium, and de-oxygenated blood from the trunk and hind limbs flows into a vein called the Inferior Vena Cava which feeds back into the right atrium.
When the heart muscles contract (systole), the heart pumps blood. The blood pressue at the time of the systole is called the systolic pressure. A pulse is the rhythmic bulging of the artery walls during systole.
When the heart muscles relax (diastole), the heart chambers are filled with blood. The blood pressure at the time of the diastole is called the diastolic pressure.
Hypertension is higher blood pressure than average. The systole and diastole refer to the blood pressure of systemic circulation. The blood pressures of pulmonary circulation and venous and arterial pressures are much smaller than that of systemic circulation.
One cycle (or beat) of contractions and relaxation is a cardiac cycle. The cardiac output is the volume of blood pumped by a ventricle per minute. The heart rate is the number of cycles per minute. The stroke volume is the volume of blood pumped per cycle.
Valves prevent backflow in the wrong direction. Atrioventricular Valves lie between each atrium and ventricle and inhibit blood from flowing from the ventricles into the atria. Semilunar Valves lie at the two ventricle exits and inhibit blood from flowing back from the arteries into the ventricles.
A set of cells in the right atrium called the pacemaker (or Sinoatrial Node) set the rate and timing when cardiac muscles contract together (coupled through gap junctions). Impulses from the sinoatrial node are delayed at the Atrioventricular Node to allow emptying of the atria completely before passing the signals on through bundle branches and Purkinje fibres throughout the rest of the heart.
An electrocardiogram (or ECG, or EKG) graphs electrical current over time measuring cardiac muscle electrical impulses as they reach the skin via body fluids.
Arteriosclerosis is the thickening, hardening and loss of elasticity of the walls of arteries.
Atherosclerosis is a type of arteriosclerosis where the blood vessel lumen accumulates fatty deposits called plaques. Ruptured plaques may cause a thrombus which leads to the damage or death of cardiac muscle cells called a heart attack (or myocardial infarction) or the damage or death of neurons called a stroke.
Neurons
Neurons (or nerve cells) are electrically excitable cells that receive, process, and transmit information through electrical and chemical signals. A ganglion is a cluster of neurons.
Nervous tissue is a tissue of neurons along with glial cells (or glia) which assist signal propagation and provide nutrients to neurons.
Dendrites are protrusions from neurons which receive signals near the main neuron cell body.
An axon is an extension of the neuron which transmits signals.
Nerves are bundles of axons.
Nervous tissue is the main component of the Central Nervous System which is made of a brain and spinal cord. The central nervous system includes the Motor System which carries signals to skeletal muscles to perform movement.
The Peripheral Nervous System includes nervous tissue besides the brain and spinal cord. The Autonomic Nervous System regulates various muscles and is generally involuntary. The autonomic nervous system is further divided into the sympathetic nervous system, parasympathetic nervous system, and enteric nervous system. The Sympathetic Nervous System contributes to arousal and energy generation in a fight-or-flight response. The Parasympathetic Nervous System contributes to the opposite, contributing to calming and returning to self-maintenance. The Enteric Nervous System contributes to regulation of the digestive tract, pancreas, and gallbladder.
There are a few major types of neurons:
- Sensory Neurons transmit information about external stimuli.
- Interneurons form local circuits within a brain or ganglion.
- Motor Neurons transmit signals to muscle cells to cause contraction and movement.
When a neuron is not signaling, it has a Resting Potential (measured in millivolts) since the inside of a neuron is negatively charged due to higher concentrations of K+ inside (and a different higher concentration of Na+ outside) which creates a voltage across the membrane. Sodium-Potassium Pumps use ATP to actively pump two K+ in and three Na+ out against their concentration gradients. There are Potassium channels which are always open to allow K+ ions to diffuse out of the cell to maintain the resting potential. There are Sodium channels which are mostly closed so that few Na+ ions diffuse into the cell. This combination of diffusions creates the resting potential. At the resting potential, even though some ion channels are always or partly open, the chemical gradient doesn’t reach equilibrium because there’s an oposing electrical gradient created by the build-up of charge; for example, as K+ ions flow out of the cell, the additional negative charge inside the cell has an attractive pull on the remaining K+ ions inside the cell. Each ion thus has an Equilibrium Potential (Eion). The Nernst Equation models the equilibrium potential of a membrane permeable to a single ion with net charge 1+ at 37°C as Eion = 62mV×(log(concentration(ionoutside) / concentration(ioninside)))
Some of the ion channels in a neuron are Voltage-Gated Ion Channels which open or close in response to a change in membrane voltage thus allowing diffusion of certain ions down their concentration gradients and thus causing a change in the membrane voltage. Hyperpolarization occurs when the inside of the neuron becomes more negative. Depolarization occurs when the inside of the neuron becomes more positive.
A temporary shift in the polarization of the membrane is called a Graded Potential which is proportional to stimulus strength.
If a stimulus depolarizes the voltage past a certain threshold, a larger change in voltage than a graded potential occurs called the Action Potential as voltage-gated Sodium channels open, letting Na+ ions into the cell resulting in further depolarization. The increased depolarization leads to additional Sodium channels opening, and thus further depolarization while most Potassium voltage-gated ion channels stay closed. The maximum voltage is constant and dependent on the composition of the Sodium ion channels in the membrane, thus the voltage magnitude of an action potential is independent of the stimulus strength (i.e. an action potential is binary: it either reaches the threshold and initiates or it doesn’t). After a certain point, voltage-gated sodium ion channels close and potassium channels open thus bringing the voltage back down. During this phase called the Refractory Period, the sodium channels are inactivated so that a second signal cannot simulataneously affect the neuron. Because of the inactivated sodium channels, the current can only move in one direction, thus a series of action potentials moves a signal along an axon as Sodium and Potassium voltage-gated ion channels open and close sequentially. Stimulus signal strength causes repeated, sequential firings of action potentials and the larger the stimulus, the more frequent the firings.
For some axons, glial cells called Oligodendrocytes in the central nervous system and Schwann Cells in the peripheral nervous system produce electrical insulation around axons called Myelin Sheaths. In such myelinated axons, voltage-gated sodium channels are only in between the myelinated sections, in areas called Nodes of Ranvier, thus signals travel faster because the action potential simply passes down the myelinated portions to the next node of ranvier in a process called Saltatory Conduction.
In Synaptic Signaling, signals pass between neurons through electrical or chemical Synapses across short gaps called Synaptic Clefts. In chemical synapses, neurons excrete Neurotransmitters which diffuse across to trigger target neurons. As an action potential arrives at the presynaptic membrane of the signaling cell, depolarization opens voltage-gated ion channels that let in Ca2+ from the outside which cause vesicles to release neurotransmitters to the target cells. At the target dendrites, Ligand-Gated Ion Channels are opened by the neurotransmitters to allow Na+ and K+ to diffuse to depolarize the membrane to create an Excitatory Postsynaptic Potential, or to allow K+ and Cl- to diffuse to hyperpolarize to create an Inhibitory Postsynaptic Potential. In the process of Summation, postsynaptic potentials in rapid succession may combine to exceed the action potential threshold at the integration center called the Axon Hillock to start another action potential. Some neurotransmitters activate G protein-coupled receptors which activate a signal cascade that opens and closes ion channels.
Example neurotransmitters include:
- Acetylcholine participates in muscle stimulation, memory formation, and learning.
- Amino acids that may act as neurotransmitters such as Glutamate which participates in long-term memory formation, and in some organisms function in the role of acetylcholine.
- Biogenic Amines which are synthesized from amino acids. Examples are Dopamine and Serotonin.
- Neuropeptides which are short peptides that may act as neurotransmitters such as Endorphins that decrease pain perception.
- Gases that may act as neurotransmitters such as Nitric Oxide (NO).
In Neuroendocrine Signaling, neurons called Neurosecretory Cells excrete Neurohormones which diffuse into the bloodstream.
Brain
A brain is an organ composed of nervous tissue that controls the rest of the organism. A spinal cord is a tubular bundle of nervous tissue used to send and receive information to and from the brain. A brain is made of three regions: The Forebrain which contains the Cerebrum and Olfactory Bulb, the Midbrain which coordinates routing sensory input, and the Hindbrain which contains the Cerebellum and controls involuntary activities.
The cerebrum (partly for voluntary thoughts and memories) is made of two parts called the left and right cerebral hemispheres which are connected by a bundle of nerves called the corpus callosum. Each hemisphere is composed of outer layers of grey matter called a cerebral cortex and inner layers of white matter. The upper surface of the cerebrum is called the pallium and some organisms have a more advanced pallium called the neocortex (partly for language).
Grey matter is different from white matter in that grey matter contains more cell bodies and fewer myelinated axons (which causes the whiteness).
Each cerebral hemisphere is made of parts called the frontal lobe (partly for dopamine processing), parietal lobe (partly for integrating sensory information), occipital lobe (partly for visual processing), temporal lobe (partly for visual memory), limbic lobe, and insular cortex (partly for consciousness). The sulci are the grooves in the brain that increase surface area.
The cerebrum is attached to the brainstem which connects to the spinal cord and is made of the pons and the medulla oblongata (partly regulating breathing, heart rate, and blood pressure).
Underneath the cerebrum and connected to the brainstem are other structures such as the cerebellum (partly for motor control), thalamus (partly for relaying signals), pineal gland, pituitary gland (partly to produce various hormones), and the limbic system.
The Pineal Gland produces the hormone Melatonin which regulates responses to light and the seasons, including sleep regulation.
Oxytocin secreted by the Posterior Pituitary Gland contracts mammary gland cells to excrete milk and influences pair bonding and sexual activity.
Prolactin secreted by the Anterior Pituitary Gland stimulates milk production.
Melanocyte-Stimulating Hormone (or MSH) secreted by the Anterior Pituitary Gland functions in hunger, metabolism, and skin coloration.
The limbic system (partly for emotions) is made of structures such as the hypothalamus (partly to link the nervous and endocrine systems, including thermoregulation and control of circadian rhythm in the Suprachiasmatic Nucleus), amygdala (partly for emotional responses), hippocampus (partly for memory), olfactory bulbs (partly for smell processing), and basal ganglia (partly for voluntary motor control).
Radial Glia are glial cells that form tracks along which new neurons migrate.
Astrocytes are glial cells that facilitate information transfer, regulate ion concentrations, promote blood flow, and help form the blood-brain barrier. The Blood-Brain Barrier is a mechanism that restricts certain substances from entering the central nervous system.
Microglia are glia cells which are immune cells.
Muscle Tissue
Muscle tissue is composed of muscle which contracts to produce force and motion. Muscle is made of parallel myocyte cells which contain the proteins actin and myosin that slide past each other to produce contraction. In Thin Filaments (similar to microfilaments), two strands of actin coil around each other. In Thick Filaments, myosin molecules are stacked on each other. Myocytes have parallel Myofibrils which are bundles of thin and thick filaments made of repeating sections called Sarcomeres which perform contractions, connected together at Z Lines, and surrounded by endoplasmic reticulum.
Skeletal muscle (or striated muscle) is attached to bones by tendons and is responsible for voluntary movements.
Smooth muscle is responsible for involuntary body movements.
Cardiac muscle performs contractions in the heart.
Movement (or motion) is the contraction of many muscle cells together:
- In a relaxed muscle, the myosin binding sites on actin are bound to long proteins running along-side actin made of Tropomyosin, and an ATP is bound to each projection on myosin called a myosin head.
- An action potential in a motor neuron releases the neurotransmitter Acetylcholine which binds to receptors on the myocyte membrane and triggers an action potential down Transverse Tubules (or T Tubules) in the myocyte. The action potential causes the myofibril endoplasmic reticulum (or Sarcoplasmic Reticulum) to release Ca2+ ions which bind to Troponin on tropomyosin which unbinds tropomyosin from the myosin binding sites on actin.
- The myosin head hydrolyzes ATP to ADP and Phosphate which extends the myosin head and binds to actin which releases the ADP and Phosphate from the myosin head which causes the myosin head to bend back down and this pulls the actin filament (called the Power Stroke).
- The simultaneous contraction of the sarcomeres shortens the myofibrils and the entire muscle cell contracts.
- ATP binds to the myosin head causing it to detach from actin and relax to its original position. As long as Ca2+ ions are present, the sequence repeats.
- When the action potential stops, Ca2+ ions go back into the sarcoplasmic reticulum, the myosin binding sites on actin are blocked, and the actin filaments relax.
A motor unit is a single motor neuron that controls a set of myocytes.
Action potential summation increases the frequency of contractions and when the rate exceeds a threshold, the tension is maximized in a contraction called Tetanic Contraction. Tonus is a partial sustained contraction in relaxed muscles. Isometric contractions increase muscle tension without increasing muscle length. Isotonic contractions change muscle length without changing muscle tension.
Powering repetitive contractions requires additional ATP by using stores of Creatine Phosphate and Glycogen within the myocyte. Muscles that rely on aerobic respiration also have Oxygen-storing protein called Myoglobin.
Fast-Twitch Fibers contract faster than Slow-Twitch Fibers because fast-twitch fibers have more sarcoplasmic reticulum.
Skeletal muscles cause movement with antagonistic contracting and relaxing muscles.
A hydroskeleton is made of fluid held under pressure in a closed body area with movement accomplished in the process called peristalsis which is rhythmic muscle activity that changes the shape of the hydroskeleton.
Senses
Senses are physical capacities that provide data about the world to the organism. A Sensory Receptor in a sensory neuron cell detects a stimulus and opens or closes ion channels creating a voltage Receptor Potential in a process called Sensory Transduction. Perception is the process in the brain of neurons processing the action potentials created by the sensory receptors.
Sensory Amplification is the process of strengthening the sensory signal during transduction. Sensory Adaptation is a decreased responsiveness of sensory receptors upon continued stimulation.
Mechanoreceptors are sensory receptors that respond to pressure, touch, stretching, motion, and sound.
Chemoreceptors are sensory receptors that respond to changes in solute concentrations and respond to certain molecules such as Glucose, Oxygen, Carbon Dioxide, and Amino Acids.
Electromagnetic Receptors and Photoreceptors are sensory receptors that respond to changes in electromagnetic energy.
Thermoreceptors are sensory receptors that respond to changes in temperature.
Nociceptors (or pain receptors) are sensory receptors that respond to extreme pressure, extreme temperature, and certain damaging chemicals.
Sensing Gravity
Gravity is sensed with mechanoreceptor organs called Statocysts which include dense material called Statoliths inside which material settles on certain ciliated cells depending on the position of the organism.
Sensing Sound and Equilibrium
Sound is a vibration that propagates as a wave of pressure through some substance such as air. An organ called an Ear has mechanoreceptor neurons called Hair Cells that transduce the pressure waves into signals in the brain. The outside of the ear is called the Pinna and sounds travel down the Auditory Canal until they are collected at the Tympanic Membrane (or eardrum). Behind the eardrum is the Middle Ear where three small bones - the Malleus (or hammer), the Incus (or anvil), and the Stapes (or stirrup) - transmit sound to the Oval Window, and opens the Eustachian Tube which equalizes pressure with the atmosphere. The bones in the middle ear transfer the sound waves to the inner ear which has fluid-filled chambers and the sounds go into the coiled cochlea bony chamber. The Cochlea has two fluid-filled canals separated by a smaller duct. The floor of the cochlear duct has the Organ of Corti which has the hair cells. Sound waves cause the membrane of the organ of corti to vibrate which bends hairs on the hair cells causing a release of a neurotransmitter across a synapse to sensory neurons.
The inner ear also has organs that sense body movement, position, and balance (or equilibrium).
Sensing Light
Light is sensed by organs called Eyes in the process of Vision.
In some organisms with Compound Eyes, there are several thousand light detectors called Ommatidia each of which have a photoreceptor.
In some organisms with eyes that have a Refractive Cornea, light enters through a transparent Cornea and into the Pupil, the diameter of which is controlled by the Iris. Behind the pupil, a Lens directs the light onto a layer of photoreceptors called the Retina (with a dip in the center called the Fovea) and the Lens is moved in or out by muscles to change Focus.
The photoreceptor cells are either cone cells or rod cells. Cone Cells detect color but are not very sensitive so act poorly in low light. Rod Cells do not detect color but are more sensitive to light so they allow organisms to see in low light in black and white. Within rod and cone cells, there is a stack of membranous disks called pigments which have a light-absorbing molecule called Retinal bound to a membrane protein called Opsin. As retinal absorbs light, it changes from a cis isomer to a trans isomer which activates opsin.
The signals of multiple photoreceptor cells are combined at Bipolar Cells, and the signals of multiple bipolar cells are combined at Retinal Ganglion Cells. Additional Horizontal Cells and Amacrine Cells help to integrate information across the retina. All of this visual information is passed to the Optic Nerve and on to the brain.
Sensing Chemicals in Solutions
Taste (or Gustation) is the sensing of chemicals (called Tastants) in a solution. An organ such as the Tongue has epithelial cells called Taste Buds which detect various types of chemicals in solutions and connect to sensory neurons.
Sensing Chemicals in Air
Smell (or Olfaction) is the sensing of chemicals (called Odorants) in the air. An organ such as the Nose has Olfactory Receptor Cells with chemoreceptors that trigger action potentials.
Gas Exchange
Gas Exchange is the process by which gases diffuse across a surface.
Breathing (or respiration, or ventilation) is a process of alternating inhalation and exhalation that facilitates gas exchange of O2 and CO2 in organs that are part of a respiratory system.
In some aquatic organisms, gills move water through the mouth and across lamellae to extract dissolved O2 and expel CO2. Some animals use countercurrent exchange to maximize gas exchange which moves substances or heat in substances in opposite directions next to each other which creates a partial pressure gradient that favors diffusion of O2 from water to blood.
In some non-aquatic organisms, in a tracheal system (network of tubes throughout a body) or in organs called lungs (localized respiratory organs), moist epithelial cells exposed to air perform gas exchange.
For lungs, air passes down a tube called the trachea (or wind pipe) and branches into one or more tubes called bronchi each of which leads to a lung. Within a lung, the bronchi branch further into bronchioles. At the tips of bronchioles, sacs called alveoli perform gas exchange. Alveoli are covered with a liquid that would produce high surface tension on the small-diameter alveoli if not for phospholipids and proteins called surfactants (or surface-active agents) coating the alveoli to reduce surface tension.
The volume of air inhaled and exhaled in one breath is called the tidal volume. The maximum tidal volume is called the vital capacity. Air that remains in the lungs after an exhalation is called the residual volume.
Positive Pressure Breathing pushes air into lungs whereas Negative Pressure Breathing pulls air into lungs. For example, in some animals, contracting rib muscles around lungs and contracting the diaphragm at the bottom of the rib cage increases the volume of the thoracic cavity which pulls in air into the lungs.
CO2 is acidic because it may react with water to form Carbonic Acid (H2CO3) which can dissociate into Bicarbonate (HCO3-) and H+. When blood pH falls, the Medulla Oblongata in the brain signals rib muscles to increase the rate and depth of breathing.
O2 is usually transported through blood or hemolymph within specialized cells called respiratory pigments which are metals bound to proteins such as Hemoglobin (a protein with quaternary structure, Fe2+ prosthetic groups, and four subunits that each may take one O2 molecule) and Hemocyanin. Low pH (e.g. high CO2) decreases the affinity of hemoglobin for O2 in an effect called the Bohr Shift.
When the level of Oxygen in the blood falls, blood vessel endothelial cells synthesize and release Nitric Oxide (NO) which diffuses into surrounding smooth muscle cells, activating an enzyme that relaxes them which increases blood flow.
In Sickle-Cell Disease, abnormal forms of hemoglobin distort the shape of red blood cells which increases the probability of lodging in arterioles, causing vessel blockage and incomplete O2 and CO2 exchange.
Adipose Tissue
Adipose Tissue (or body fat) is a loose connective tissue that stores triglycerides, cushions an organism, produces heat, and produces hormones. Adipose tissue is composed mostly of adipocyte cells along with some other cell types. White adipose tissue stores triglycerides. Brown adipose tissue generates heat.
Fatty acid degradation
Lipolysis (or Fatty Acid Degradation) is the process of converting stored fat into Acetyl-CoA which enters the Citric Acid Cycle to produce energy. Lipolysis may occur when Glucose levels are low:
- Hormones such as Epinephrine and Glucagon are released which bind to receptors on adipocytes that activate perilipin proteins on the surface of lipids.
- Phosphorylation of perilipins causes phosphorylated hormone-sensitive lipase to hydrolyze triglycerides in the lipid droplets which produces glycerol and fatty acids.
- The fatty acids exit the adipocyte, bind to the plasma protein serum albumin, and enter the blood. Glycerol also enters the blood and travels to the liver.
- Fatty acids then enter cells and are attached to CoA to create Acyl-CoA.
- A Carnitine is added to Acyl-CoA to create Acyl-Carnitine.
- Acyl-Carnitine enters the mitochondria and is broken down into Acetyl-CoA through a process called beta oxidation (or β-oxidation).
- Acetyl-CoA enters the Citric Acid Cycle.
Lipoproteins
Lipoproteins are used to transport hydrophobic lipids (such as Cholesterol) through the circulatory system. A lipoprotein is a set of lipids wrapped with a phospholipid bilayer with embedded proteins called Apoproteins.
Lipoproteins are categorized by their relative densities of lipids to proteins from lowest to highest: Chylomicron, Very Low-Density Lipoprotein (VLDL), Low-Density Lipoprotein (LDL), and High-Density Lipoprotein (HDL).
Endothelial cells in the walls of the capillaries of adipose and muscle tissue have an enzyme called Lipoprotein Lipase (LPL) which interacts with Apoprotein C-II on chylomicrons to hydrolyze the packaged triglycerides into glycerol and fatty acids and send them into adipose and muscle cells. The remaining chylomicrons are transported to the liver and cleaned up in a process mediated by Apoprotein E.
Liver
The liver (hepat in Greek) is an organ with hundreds of functions such as detoxification, regulating Glucose and Glycogen, producing hormones, decomposing red blood cells, and producing bile. The liver is made mostly of Hepatocyte cells.
The liver may synthesize lipoproteins such as LDL and VLDL which carry cholesterol and triglycerides to tissues.
HDL transports cholesterol from tissues to the liver.
Growth Hormone (or GH) is released by the Anterior Pituitary Gland and stimulates growth of the organism, primarily by causing the liver to release Insulin-Like Growth Factors (or IGFs) which stimulate bone and cartilage growth.
Pancreas
The pancreas is an organ that releases various hormones into the blood and various digestive enzymes into the small intestine.
Fluid Waste
Some organisms create a fluid waste called urine which is excreted. In the process of filtration, blood or hemolymph interact with transport epithelia and due to their liquid pressure (e.g. blood pressure), water and small solutes cross the epithelial membrane into an excretory tubule, forming the urine solution called filtrate. Some non-wastes are recovered back into the blood or hemolympy through a process of reabsorption. Additional wastes from capillaries neighboring the excratory tubule enter the filtrate through a process of secretion.
Kidney
The Kidneys are organs which filter blood to eliminate waste and excess salt while conserving water. Urine exits a kidney in a tube called the ureter and drains into the urinary bladder. Urine is excreted from the bladder and through the urethra in the process of urination.
The outside of a kidney is the renal cortex within which is the renal medulla and both are supplied with blood by a renal artery which interacts with excretory tubules called nephrons and juxtamedullary nephrons to produce urine filtrate which is collected in the renal pelvis before leaving in the ureter to the bladder.
Nephrons are made of a ball of capillaries called the glomerulus, an excretory tubule called the proximal tubule, and a part of the tubule which encapsulates the glomerulus called Bowman’s Capsule. Nephrons are supplied with blood from afferent arterioles. Blood pressure in the glomerulus creates filtrate in Bowman’s Capsule and the filtrate passes through the proximal tubule to the Loop of Henle wrapped with arterioles called the vasa recta, and then the Distal Tubule before entering collecting ducts in the renal pelvis. As filtrate descends the Loop of Henle through the medulla, water is extracted through aquaporins and increases in solute concentration. As filtrate moves up the Loop of Henle, salt diffuses into the interstitial fluid. The nephrons are countercurrent multiplier systems which create an osmolarity gradient for extracting water from filtrate in the collecting duct.
Urine volume and osmolarity are regulated in response to the availability of salt and water. If salt is ingested or water is lost from the body, then blood osmolarity increases. Over a certain blood osmolarity threshold, the hypothalamus triggers release of Antidiuretic Hormone (or ADH, or vasopressin) from the Posterior Pituitary Gland which binds to collecting duct cells that activate a signal transduction cascade that adds aquaporin proteins into the cell membrane which enhance reabsorption of water from the collecting duct.
If both salt and water are lost from the body, the Renin-Angiotensin-Aldosterone System (or RAAS) also regulates kidneys. A tissue called the Juxtaglomerular Apparatus (or JGA) around the afferent arterioles releases the enzyme Renin that creates a hormone Angiotensin II which triggers vasoconstriction and the release of the hormone Aldosterone which causes the collecting duct cells to reabsorb more water and sodium.
In response to an increase in blood volume and pressure, the walls of the atria of the heart release the hormone Atrial Natriuretic Peptide (or ANP) which inhibits the release of Renin from the JGA, inhibits reabsorption of salt, and inhibits Aldosterone release.
When O2 levels fall, the kidneys secrete a hormone called Erythropoietin (or EPO) that stimulates the creation of more erythrocytes.
Adrenal Glands
The Adrenal Glands exist on top of the kidneys. The adrenal glands are made of the outer Adrenal Cortex and inner Adrenal Medulla.
Under stress of physical threat, exercise, or cold exposure, the hypothalamus activates the adrenal medulla via nerve impulses (the fight-or-flight response) which excrete the hydrophilic hormones Epinephrine (or Adrenaline) and Norepinephrine (or Noradrenaline) which are Catecholamines. Catecholamines increase the rate of Glycogen breakdown in the liver and skeletal muscles, increase the release of Glucose in the liver, increase Lipolysis in adipose tissue, increase heart rate and stroke volume, change blood vessel constriction and dilation to direct more blood to the heart, brain, and skeletal muscles, and dilate the bronchioles in the lungs.
Under stress of low blood sugar, decreased blood pressure or volume, or shock, the hypothalamus releases a hormone which stimulates the Anterior Pituitary Gland to release Adrenocorticotropic Hormone (or ACTH) which stimulates the adrenal cortex to secrete Corticosteroids. Glucocorticoids are corticosteroids which promote Glucose synthesis from noncarbohydrate sources and promote skeletal muscle to break down protein into amino acids for conversion into Glucose in the liver. Mineralocorticoids are corticosteroids which help maintain salt and water balance.
Thyroid Gland
The Thyroid Gland is an organ in the neck with two lobes on top of the trachea. The thyroid gland produces Thyroid Hormones which help with regulating metabolism, maintaining normal blood pressure, heart rate, and muscle tone, and regulating digestive and reproductive functions.
When the levels of thyroid hormones drop below a threshold, the hypothalamus releases Thyrotropin-Releasing Hormone (or TRH) which causes the Anterior Pituitary Gland to release Thyrotropin-Stimulating Hormone (or TSH, or Thyrotropin) which stimulates the thyroid gland to secrete Thyroid Hormones. Thyroid Hormones are Triiodothyronine (or T3) which contains three Iodine atoms and Thyroxine (T4) which contains four.
If Ca2+ levels increase beyond a threshold, the thyroid gland releases Calcitonin hormone which inhibits bone breakdown and increases Ca2+ secretion in the kidneys.
Parathyroid Glands
The Parathyroid Glands are four small structures on the surface of the thyroid. If Ca2+ levels drop below a threshold, the Parathyroid Glands produce Parathyroid Hormone (or PTH) which causes the increase of Ca2+ in the blood by causing breakdown of bone into Calcium and reabsorption of Calcium in the kidneys.
Gonads
Gonads are organs that produce gametes and hormones related to sexual reproduction.
Sex Hormones contribute to growth, development, reproductive cycles, and sexual behavior.
Gonads in males are called the Testes. Testes create gametes called Sperm in coiled tubes called Seminiferous Tubules. Testes drop into sacs outside the main part of the body called Scrotums. A testis within a scrotum is a Testicle. Sperm pass through the Seminiferous Tubules into a collecting duct called the Epididymis. During ejaculation, sperm are expelled from the Epididymis through a muscular tube called the Vas Deferens which opens into the Urethra and exit through the Penis. Sperm are ejected in a fluid called Semen which is created by Seminal Vesicles, the Prostate Gland, and the Bulbourethral Glands. The tip of the penis is called the Glans (or head) and is surrounded by a layer of skin called the Prepuce (or foreskin). Sperm have a head called the Acrosome which has enzymes that help sperm penetrate an egg.
Testes produce Androgen Hormones such as Testosterone which affect male development. In general, females also produce androgens but in a much smaller amount.
Gonads in females are called Ovaries. The outer layer of ovaries include Follicles which consist of an Oocyte that is a partially developed egg. Tubes called Oviducts (or fallopian tubes) extend from the ovaries into an organ called the Uterus (or womb) where zygotes develop. Fertilization of sperm and egg occurs in the fallopian tubes. Cilia in the oviducts move eggs down into the uterus. The inner lining of the uterus is called the Endometrium. The bottom of the uterus is called the Cervix and opens into a muscular and elastic chamber called the Vagina. The vagina opens into the Vulva which describes all external female genitalia, including thick skin folds on the outside called the Labia Majora, slender skin folds on the inside called the Labia Minora, and the Clitoris which has a glans and prepuce.
Some organisms have cycles of reproductive activity controlled by hormones related to seasons or an internal clock so that reproduction only occurs in favorable conditions. In females, during the Ovarian Cycle, Ovulation occurs in the midpoint of a reproductive cycle to release mature eggs available for fertilization. Changes in the uterus define the Uterine Cycle which in some organisms is called the Menstrual Cycle wherein the endometrium thickens with additional blood supply. In some organisms, if an egg is not fertilized, a process called Menstruation excretes the excess endometrium out of the body in preparation for the next cycle, whereas in other organisms with an Estrous Cycle, the excess endometrium is not excreted. The menstrual cycle stops working permanently at a time in life called Menopause.
Ovaries produce Estrogen Hormones such as Estradiol and Progesterone which affect development and the support and growth of an embryo. In general, males also produce estrogens but in a much smaller amount.
The hypothalamus secretes Gonadotropin-Releasing Hormone (or GnRH) which causes the Anterior Pituitary Gland to secrete the gonadotropins Follicle-Stimulating Hormone (or FSH) and Luteinizing Hormone (or LH) which regulate activity of the gonads.
Mammary Glands
The Mammary Glands, while present in both sexes, only produce milk (for nurturing children) in females.
Nutrition
Nutrition is the ingestion, digestion, and usage of food by an organism.
Essential Nutrients are substances required to be ingested because they cannot be synthesized with simpler molecules. Essential Amino Acids are any amino acids that cannot be synthesized by the organism. Essential Fatty Acids are some fatty acids that cannot be synthesized such as Linoleic Acid.
Vitamins are small organic molecules required in metabolism. Some vitamins cannot be synthesized so they are essential nutrients. Some vitamins are water soluable and some are fat soluable.
Minerals are inorganic, solid chemical compounds such as Iron and Sulfer which are required in metabolism by some organisms.
Auxotrophy is the inability of an organism to synthesize all of the nutrients and vitamins required for survival. Prototrophy is the ability of an organism to synthesize all of the nutrients and vitamins required for survival.
Ingestion
The mouth (or Oral Cavity) ingests food. A fluid called Saliva is created by the salivary glands. Saliva includes mucus which lubricates and pre-processes food.
Food is balled up into a complex called a bolus. The throat is connected to the mouth and leads either to the muscular tubes called the trachea that leads to the lungs or the esophagus that leads to the stomach. The bolus is passed into the pharynx which is a region of the throat right before the split into the two different tubes into the lungs or stomach. Swallowing is the process of passing the bolus from the mouth to the esophagus using contractions.
Material moves through the esophagus and other areas with the process of peristalsis.
At the end of the esophagus, food reaches a sphincter which is a muscle that regulates passage into the stomach.
Digestion
Digestion is the breakdown of food into small, water-soluble nutrients (e.g. amino acids, sugars, etc.) in the blood and non-water soluble molecules (e.g. lipids, etc.) in the lymph.
Digestion occurs in the Gastrointestinal Tract (or GI tract, or Alimentary Canal, or gut) which includes the mouth, esophagus, stomach, small intestine, large intestine, and anus.
The stomach is an organ that stores food and starts its processing. As food stretches the walls of the stomach, the hormone Gastrin is released which causes the secretion of gastric acid to begin food digestion. Gastric acid is made of Hydrochloric Acid (HCl) and Pepsin (synthesized with HCl from Pepsinogen) and has a pH of about 2 which denatures proteins and kills most bacteria. Pepsin is a protease which breaks down proteins. HCl is produced by parietal cells, pepsinogen is produced by chief cells, and mucus is produced by mucous cells. The partly digested, acidic collection called Chyme is passed onto the small intestine.
The small intestine completes most food digestion. The Duodenum is the first part of the small intestine which completes the breakdown of the chyme using juices from the pancreas, liver, and gallbladder. The entry of chyme into the small intestine stimulates secretion of the hormone Secretin which stimulates the pancreas to secrete Biocarbonate into the duodenum which acts as a buffer for digestion. The pancreas also secretes the proteases Trypsin and Chymotrypsin into the duodenum.
The Jejunum is the second part of the small intestine which absorbs nutrients into the blood stream through projections called villi on epithelial cells called enterocyte cells. The villi have further microvilli projections that increase surface area. Water-soluble nutrients enter the blood into the hepatic portal vein which leads to the liver which transforms and filters some nutrients and sends them in blood to the heart which pumps them to the whole body.
The Ileum is the third part of the small intestine which absorbs any remaining Vitamin B12, bile acids, ions and most water.
The ileum connects to the large intestine (including the colon, cecum, and rectum) at a T-shaped junction. One branch of the T junction leads to the cecum which ferments some ingested material and includes an extension called the appendix which may have symbiotic microorganisms. The other branch of the T junction leads to the colon which completes the recovery of most water and the remaining material is called feces which are driven to the rectum. The rectum is the end of the large intestine where feces are stored until expulsion. A sphincter at the end of the rectum controls expulsion of feces through the anus.
Diarrhea is a condition where a human has at least three loose, liquid, or watery bowel movements per day. Diarrhea may cause dehydration (excessive water loss).
The collection of microorganisms living in and on the body is called the microbiome and some bacteria in the intestines help digest food and produce vitamins. Some bacteria such as some strains of Escherichia coli (E. coli) are commensal and secrete antibacterial proteins called bacteriocin (e.g. colicins) that crowd out parasitic bacteria. After long treatments of antibiotics which may kill many of the commensal microbiota, disease-causing bactera such as Clostridium difficile (C. difficile) may establish themselves and secrete toxins that cause diarrhea or pseudomembranous colitis. There is little Oxygen after the first few inches of the gut, so any remaining bactera are anaerobic.
The average human has about 1000 species of bacteria living in the gut, particularly because it’s a sort of constant food. Approximately half of the dry weight of feces is commensal bacteria (1011 bacteria per gram).
Probiotics are live microorganisms ingested to try to improve digestive microbiota. Probiotics may be effective for acute infectious diarrhea, antibiotic-associated diarrhea, Clostridium difficile-associated diarrhea, hepatic encephalopathy, ulcerative colitis, irritable bowel syndrome, functional gastrointestinal disorders, and necrotizing enterocolitisa.
Bile Acid
Bile acid is secreted in the duodenum and is used for digestion and absorption of fat from food. Bile acid emulsifies and breaks apart fat and lipid complexes. Bile acid is a large part of bile which is secreted by the liver and stored and concentrated in the gallbladder.
Lipid Digestion
A lipase is an enzyme that hydrolyzes fats. Dietary triglycerides and cholesterol are hydrolyzed by lipases in the stomach and duodenum into monoglycerides and free fatty acids (fatty acids bound to an albumin protein), absorbed by enterocytes, recombined into triglycerides, coated with phospholipids, cholesterol and proteins into water-soluble chylomicrons, and sent into lacteal vessels that are part of the lymphatic system, and ultimately into blood pumped by the heart to the rest of the body.
Carbohydrate Digestion
α-Amylase breaks down starch into maltose (and other small carbohydrates).
Protein Digestion
Carboxypeptidase is produced in the pancreas.
Nitrogenous Waste
A byproduct of protein and nucleic acid digestion is ammonia which may be toxic. Organisms have different ways of dealing with nitrogenous waste:
- Excreting ammonia (most common in aquatic organisms).
- Converting ammonia to Urea which is ammonia combined with CO2 in the liver.
- Converting ammonia to Uric Acid.
Blood Sugar
When blood glucose levels rise above a certain level, the hormone Insulin is secreted by Beta Cells in the pancreas which triggers cells to uptake Glucose, and liver and muscle cells may store Glucose in the form of Glycogen.
When blood glucose levels decrease below a certain level, the hormone Glucagon is secreted by Alpha Cells which triggers the release of Glucose from Glycogen stores.
Diabetes Mellitus is a disease in which insufficient insulin is produced (Type 1 Diabetes) or cells have a decreased response to insulin (Type 2 Diabetes).
Appetite
Appetite is the feeling of hunger or the opposite of hunger, satiety. The hormone Ghrelin secreted by the stomach wall triggers feelings of hunger. Insulin, the hormone Leptin produced by adipose tissue, and the hormone PYY produced by the small intestine after meals trigger feelings of satiety.
Glands
Glands are groups of cells that release chemicals either into the blood (endocrine gland) or from epithelial cells on the inside or outside of the body (exocrine gland).
Invertebrates
Most animals are invertebrates which are animals that do not have a backbone and secrete an exoskeleton made of Calcium Carbonate and Chitin.
Insects are six-legged (hexapod) arthropods, many of which can fly without sacrificing walking legs. Many insects undergo metamorphosis. In holometabolism (or complete metamorphosis), organisms first have a larval stage (e.g caterpillar, maggot, grub) specialized for eating and growing, followed by a pupal stage specialized for reproduction and dispersal. In hemimetabolism (or incomplete metamorphosis), young organisms look like adult organisms, but go through various stages of molting.
Vertebrates
[Chordates][] are animals with a notochord which is a flexible rod between the digestive tube and nerve cord, and provides skeletal and muscle support.
Vertebrates are chordates in which the notochord develops into a series of bones called a backbone (or vertebral column or spine) made of bones called vertebrae which house the spinal cord and allows for more complex movement.
Vertebrates called Gnathostomes have jaws which are hinged structures in the mouth with teeth that help to grip and slice food.
The three main classes of vertebrates are amphibians, reptiles, and mammals.
Amphibians, in general, develop first in water (tadpole stage) and then go through metamorphosis (which involves an abrupt change in body type) to live on land.
Tetrapods are vertebrates with four limbs well adapted for movement on land. A limb is an organ that protrudes from the body and is usually used to aid in movement and other functions.
Amniotes are tetrapods that develop an egg adapted for land conditions. The amnion is a fluid-filled cavity that protects against shock of movement. The yolk sac contains yolk which are nutrients for the embryo.
Reptiles are tetrapods that have scales made of the protein keratin for protection and lay shelled eggs on land. Most reptiles are ectothermic, meaning they regulate their body temperature using external heat, although birds are endothermic, meaning they regulate their body temperature through metabolism. Birds are reptiles adapted for flight.
Synapsids are animals that have holes in their skull (those with two holes are diapsids) allowing for muscles to control the jaw. The earliest synapsids existed about 300 million years ago.
Therapsids evolved from synapsids and have specialized teeth called canines for piercing. The earliest therapsids existed about 280 million years ago.
Mammals are amniotes evolved from therapsids and usually have mammary glands that produce milk for offspring and hair. The earliest mammals existed about 145 to 200 million years ago.
Mammals use the XY sex determination system where the two allosomal chromosomes in females are the same (two chromosomes called X) and the allosomal chromosomes of males are different (one X chromosome and one chromosome called Y). In females, a random one of the X chromosomes deactivates into an object called a Barr body (this occurs independently in multiple embryonic cells, thus some females have mosaicism of some traits).
Primates are mammals that have hands and feet adapted for grasping. Monkeys and apes (anthropoids) are primates that have an opposable thumb that allows for more advanced grasping.
Humans
Humans (or Homo Sapiens) are apes that stand upright, have bipedal movement and a large brain, language, use of complex tools, etc. The human species is considered about 200,000 years old. Closely related species of the past are called hominins, going back at least 6.5 million years, although the more specific Human genus Homo goes back at least 1.6 to 2.4 million years.
About 2% of the human genome encodes for proteins.
Most human enzymes act optimally at a temperature of ~37 °C / ~98.6 °F.
The average diameter of a human cell is a few dozen µm. An average human body has about 1027 atoms.
Animal Behavior
A stimulus transmitted from one organism to another is called a Signal. The transmission and reception of signals between organisms is called Communication. Organisms may communicate which each other through odor and taste using chemical signals called Pheromones.
Animals have various behaviors that are movements in response to stimuli. A simple Sign Stimulus may cause a behavior called a Fixed Action Pattern. Some behaviors such as fixed action patterns, pheromone signaling, etc., are approximately the same across a species and do not require previous experiences and are called Innate Behaviors (or Instincts).
Learning is the process of changing behavior by using Memories which store information about experiences and thus such behaviors are not innate. In some species, learning called Imprinting is the establishment of a long-lasting behavior in response to some organism or object during an early phase of life called the Sensitive Period (or Critical Period). Spatial Learning is learning an environment’s spatial structure (e.g. memories of objects in space). Associative Learning is learning which associates a feature of an environment (e.g. color) with some meaning (e.g. bad taste). Social Learning is learning by observing other organisms which is a large part of what creates a Culture. The most advanced forms of learning involve Cognition which is the process of Knowing using awareness, reasoning, recollection, problem solving, and judgment.
Activities that only occur during the day time are diurnal.
A long-distance change in location is called Migration.
Foraging is food-obtaining behavior, including searching, recognition, and capture.
In Promiscous Mating, no long-term bonds form between mating males and female. In Monogamous Mating, a male and a female may form a longer-term relationship. In Polygamous Mating, an individual of one sex and multiple individuals of the other sex form a longer-term relationship (in Polygyny, a single male and in Polyandry, a single female). Mate-Choice Copying is a form of social learning in which sexual selection is partly driven by the sexual selection of other individuals.
An individual animal’s behavior is Altruistic if it reduces its fitness but increases the fitness of other individuals (e.g. parents sacrificing for children). Hamilton’s Rule (natural selection favors altruism if r×B > C) is a hypothesis that altruism is related to the benefit to the recipient (B), the cost to the altruist (C), and the coefficient of relatedness (r; the average fraction of shared genes). Hamilton’s Rule predicts the hypothesis of Kin Selection in which altruism is higher amongst relatives (Kin). Altruism amongst individuals that are unrelated or weakly related is called Reciprocal Altruism.
Lekking is a behavior in some organisms in which males gather in an area and compete for access to females.
Defense Mechanisms
A Predator is an organism that kills and eats a Prey organism. An Apex Predator is a predator that is not prey to predators of other species within its environment.
Some organisms have defense mechanisms against predators. Aposematic Traits are colors, sounds, or odors that signal to predators that they may have traits that will hurt them. Masting occurs in some plants which produces a large number of germinated offspring at the same time to exceed predation capacity.
Protists
Protists are something of a catch-all name of mostly unicellular (and some multi-cellular) eukaryotic organisms which don’t fit into other kingdoms (i.e. not animals, plants, or fungi).
The phylogeny of protists is roughly:
- Excavata: Similarities in exoskeleton and most have an excavator feeding groove.
- Diplomonads: Contain mitochondria without electron transport chains called mitosomes.
- Parabasalids: Contain mitochondria called hydrogenosomes which release Hydrogen gas as a by-product of anaerobic respiration.
- Euglenozoans: Presence of a rod inside flagella. Includes Euglenids and Kinetoplastids which contain a single, large mitochondrion with mtDNA in a kinetoplast.
- SAR
- Stramenopiles: Most include flagella with hair.
- Diatoms: Unicellular algae with a strong silicon dioxide cell wall.
- Golden Algae: Golden color from yellow and brown carotenoids.
- Brown Algae: Brown or olive color from plastids. Some include plant-like components such as the holdfast for anchoring, the stemlike stipe, and leaflike blades. Includes Kelp.
- Alveolates: Have sacs under the plasma membrane called alveoli.
- Dinoflagellates
- Apicomplexans: Include Plasmodium which is the parasite that causes Malaria.
- Ciliates
- Rhizarians
- Radiolarians
- Forams
- Cercozoans
- Stramenopiles: Most include flagella with hair.
- Archaeplastida
- Red Algae
- Green Algae
- Chlorophytes
- Charophytes
- Plants (non-Protists)
- Unikonta
- Amoebozoans
- Slime Molds
- Tubulinids
- Entamoebas
- Opisthokonts
- Nucleariids
- Choanoflagellates
- Fungi (non-Protists)
- Animals (non-Protists)
- Amoebozoans
Cell Death
Cell death is either caused by necrosis or a process of programmed cell death.
Necrosis is a cell injury caused by extracellular factors (e.g. trauma, toxins, etc.) which leads to autolysis (or self-digestion).
Programmed cell death is the regulated death of a cell proximately using intracellular mechanisms such as apoptosis, autophagy, and necroptosis.
Apoptosis is a form of programmed cell death in which processes such as signal transduction instruct the cell to commit suicide.
Autophagy is a form of programmed cell death in which certain signals lead to the creation of autophagosomes which are double-layered vesicles that consume parts of a cell and which are ultimately fused with lysosomes for degradation.
Necroptosis is a programmed form of necrosis and may be an alternative or backup to apoptosis.
Etcetera
Heat-shock proteins in both animals and plants are synthesized in response to a temperature over a threshold and help protect other proteins from denaturing.
Viruses
Viruses (singular viron) have DNA (single- or double-stranded) or RNA (single- or double-stranded) but do not have a nucleus or other organelles. Instead, viruses surround their genome with a protein shell called a capsid (made up of proteins called capsomeres) with glycoproteins called attachment fibers to help bind with surface proteins on a target cell. Viruses may have an additional viral envelope around the capsid which is a phospholipid bilayer plasma membrane with glycoproteins called spikes used for attachment. Viruses reproduce by injecting their genes into an existing cell and use the cell’s machinery to reproduce. Viral DNA or RNA may be called vDNA or vRNA, respectively. Viruses are generally about 10 - 300nm in diameter. Virus shapes are generally either helical, polyhedral, or complex.
Viruses that infect bacteria are called bacteriophages (or phages). The capsid on a phage is connected to a tail structure that helps to attach to a target cell, after which enzymes digest part of the membrane to allow the phage to insert its genes.
A lytic virus causes a cell to ultimately burst and die, whereas a lysogenic virus replicates the virus without destroying the cell. An enveloped virus uses exocytosis to pick up the plasma membrane from the cell to use as its envelope.
Types of viruses:
- Type I: Double-Stranded DNA (dsDNA)
- Type II: Single-Stranded DNA (ssDNA)
- Type III: Double-Stranded RNA (dsRNA)
- Type IV: Positive-strand Single-Stranded RNA (ssRNA) which serves as mRNA
- Type V: Negative-strand Single-Stranded RNA (ssRNA) which uses a polymerase (packaged with the virus) to create the positive-strand ssRNA that serves as mRNA
- Type VI (e.g. Retrovirus): Single-Stranded RNA (ssRNA) which uses reverse transcriptase to create dsDNA
DNA viruses tend to evolve more slowly because of DNA proof-reading, whereas RNA viruses tend to be more adaptable because of replication mistakes (antigenic drift).
Examples of viruses:
- Influenza virus (causes the flu): Lytic virus that uses Hemagglutinin glycoproteins on the viral envelope (attachment spikes) to bind to Sialic Acid sugars on the surface of epithelial cells, and Neuraminidase which is an enzyme that breaks the extracellular matrix of some cells. Negative-sense (3’ to 5’) vRNA and proteins such as RNA replicase are injected into a target cell and transported into the cell nucleus. RNA replicase transcribes the vRNA into positive-sense (5’ to 3’) viral mRNA which starts the normal protein creation process. Other viral proteins degrade cellular mRNA and produce additional negative-sense vRNA for additional virons that exit through exocytosis.
- Human Immunodeficiency Virus (or HIV): Infects certain white blood cells. HIV is a retrovirus which uses reverse transcriptase to create DNA once inside the white blood cell and this DNA is permanently integrated into the cell’s DNA (these viral integrated genes are called a provirus) and then creates new HIV viruses.
CRISPR-Cas
Clustered Regularly Interspersed Short Palindromic Repeats (CRISPRs) are repetitive DNA sequences in some prokaryotes that code for parts of certain phage DNA. Nucleases called Cas (CRISPR-associated) proteins (e.g. Cas9) use RNA transcribed from CRISPR sequences to identify and cut phage DNA thus inactivating the phage.
Prions
Prions are proteins which may infect organisms (e.g. through food) and cause brain diseases such as Mad Cow Disease, Creutzfeldt-Jakob Disease, and Scrapie. The prion proteins are misfolded proteins which may cause properly folded proteins of the same type to misfold upon contact which may lead to a chain reaction.
Birth
Birth is the process of one organism bearing a separate child organism.
An oviparous animal develops eggs into a shelled container with yolk for nourishment and the egg develops and hatches mostly outside the mother. A viviparous animal develops eggs inside the mother (gestation or pregnancy) with nutrient exchange between egg and mother before birth. An ovoviviparous animal develops eggs inside the mother until they are ready to hatch, but which are self-sufficient with egg yolk and without nutrient exchange with the mother.
After fertilization between sperm and egg creates a zygote, the zygote starts to perform mitosis to create the embryo, enclosed within the outer membrane of the egg called the zona pellucida. This initial mitosis is called cleavage in which the number of cells (called blastomeres) increases but the amount of cytoplasm stays the same, thus each blastomere is smaller than its parent cell.
In some animals, the egg is partly composed of stored nutrients called the yolk which is concentrated to one pole of the egg called the Vegetal Pole and the other cells concentrate at the other end called the Animal Pole. In some animals such as frogs, after fertilization, the contents of the animal pole shift slightly towards the point at which the sperm entered thus creating a crescent shaped area between the poles with diffuse pigments that look gray called the gray crescent.
Once cleavage creates ~16 cells which are grouped into a solid ball, the collection of cells is called a morula. Cells on the outer part of the morula become trophoblast cells and compact together with gap junctions and desmosomes. The remaining cells on the inside are called the embryoblast (or Inner Cell Mass).
The zygote and blastomeres are totipotent stem cells because they can differentiate into the cells of the body and the cells outside the body (e.g. trophoblasts). The cells of the embryoblast are called embryonic stem cells and they’re pluripotent meaning that they may differentiate into the cells of the body.
Trophoblast cells induce the creation of a fluid-filled cavity (using Na+ ions and osmosis) called the blastocoel inside the morula which contains the embryoblast and the collection of cells is now called the blastula (or blastocyst). In mammals, the trophoblasts of the blastocyst implant into the endometrium which is the inner epithelial layer of the mother’s uterus, and this implantation creates the placenta organ which allows for growth, nutrient exchange, waste disposal, etc.
The cells of the blastula differentiate into multiple different layers and the total collection of cells is called the gastrula. The layers include the ectoderm (outermost) and endoderm (innermost) in diploblastic organisms and include a third mesoderm layer (middle layer) in triploblastic organisms. There is also an opening called the blastospore which becomes the mouth or anus, and intestinal lining.
The differentiation of the gastrula continues into organogenesis in which the layers of cells differentiate to start to create various organs.
The embryo is called a fetus when all majory body organs are present.
A sac forms around the fetus called the amniotic sac which contains the fetus and amniotic fluid and is connected to the placenta. The membrane of the amniotic sac is called the amnion.
The amniotic sac is further enclosed by a membrane called the chorion which has cells called chorionic villi on the outside that interact with the uterus for nutrient exchange.
In Semelparity, an organism reproduces once and then dies. In Iteroparity, an organism may reproduce multiple times.
There is a trade off between number of offspring and the amount of resources parents may devote to each offspring. Selection for traits that are advantageous at high density is called K Selection. Selection for traits that are advantageous at low density is called R Selection.
Precocial Species are those in which children are relatively mature and mobile when born, whereas Altricial Species require significant nursing after birth.
Planetary Science
Planetary Science is the study of planets. The Earth’s orbit around the sun is an elliptic orbit (also called ecliptic) at ~30km/s with an average distance to the sun of ~150 billion meters (bm). Although arbitrary, the Earth and Sun are generally viewed from the vantage point where the Earth orbits the sun in a counter-clockwise direction (Eastward), and the Earth and Sun themselves spin in a counter-clockwise direction around their North-South axes. From this vantage point, there are two opposite poles for the Earth: the North Pole and the South Pole. It takes about one day (24 hours) for the Earth to make a single rotation around its axis. It takes about 365.256 days for the Earth to make a single orbit around the sun. Earth’s North-South axis is tilted about 23.44° from the orbital plane (towards Polaris - the Northern Star). The moon rotates counterclockwise around the Earth and itself rotates counterclockwise around its North-South axis.
A location on the Earth is described by a latitude and a longitude, measured in degrees (°). The equator is the mid-point between the North and South-poles which divides the Earth into the Northern Hemisphere and Southern Hemisphere. The length of the equator - the circumference of Earth - is approximately 40,075 km.
Latitude specifies the North-South position of a point relative to the equator in degrees and a North (N) or South (S) designation. Therefore, the equator is at 0°, the North Pole is at 90° N, and the South Pole is at 90° S. Latitude may also be called a parallel.
Longitude specifies the East-West position of a point relative to the Prime Meridian - which passes through Greenwich, England - in degrees and a East (E) or West (W) designation. Therefore, Greenwich is at 0°, and a point West of Greenwich can range up to -180° (or 180° W) or up to 180° (or 180° E).
A location is normally specified with latitude first and longitude second. Latitude and longitude may be specified in decimal form (e.g. 40.771850, -73.974850 for New York City) or in degrees, minutes, and seconds of an arc (e.g. 40°46’18.7”N, 73°58’29.5”W New York City).
There are four other common parallels of reference: the Arctic Circle at 66.57° N, the Tropic of Cancer at 23.43° N, the Tropic of Capricorn at 23.43° S, and the Antarctic Circle at 66.57° S. The areas below the tropic of cancer and above the tropic of capricorn (including the equator) are called the tropics. The areas between the tropic of cancer and 35°N, and the areas between the tropic of capricorn and 35°S are the subtropics. The areas between the subtropics and the polar circles are called the temperate zone.
More solar radiation reaches low latitudes (near the equator) than high latitudes (near the poles) because it hits the higher latitudes at an angle.
A solstice is a day of a year in the Earth’s orbit around the sun when the tropic of cancer and the tropic of capricorn have the most or least amount of sunlight relative to other days in the year. During the June solstice (approximately June 21), the tropic of cancer receives the most and the tropic of capricorn receives the least. During the December solstice (approximately December 21), these are flipped. The hemispheres have opposite behavior because of the Earth’s tilt.
An equinox is a day of a year in the Earth’s orbit around the sun when the equator has the most amount of sunlight relative to other days in the year and all places on Earth have an equal day and night period. There are two such days: the Vernal equinox (approximately March 21) and the Autumnal equinox (approximately September 23).
The amount and duration of sunlight are directly related to the temperature of different parts of Earth and a year is broken down into some number of general seasons (depending on the latitude and logitude) such as summer (hottest season) and winter (coldest season).
Weather is the state of the atmosphere such as temperature. Climate describes long-term weather trends.
From the surface of Earth, the atmosphere decreasess in temperature as altitude increases until the Tropopause (below which is considered the Troposphere - up to between 6-18km), after which temperature increases through the Stratosphere due to absorption of solar radiation in the Ozone Layer. The troposphere is where most weather occurs. Above the stratosphere is the Mesosphere, after the stratopause, where temperature again decreases as altitude increases.
The tropopause causes water vapor to cool and condense into rain. This tends to happen in areas of higher heat and lower pressure as the warm air rises and hits the troposphere. Areas of lower temperatures have higher pressure and tend to experience dry, clear conditions.
Warm air is less dense than cold air. Moist air is less dense than dry air because H2O is less dense than air (N2, O2, Ar, CO2, etc.). Wind is a flow of air from high pressure to low pressure.
In general, there is a temperature gradient from the poles (cold) to the equator (warm) due to solar radiation. Therefore, on a non-spinning Earth, wind would tend to go from the poles to the equator due to the pressure differential. However, because the Earth is spinning and the air is not solidly attached to the Earth, the Coriolis Effect (the change in velocity with latitude) causes the air, in general, to be deflected to the West as it moves towards the equator. Winds are categorized into a few broad categories and they are named based on where the air particles start. The tropics and subtropics (to about 30° - the horse latitudes) form the trade winds (North-East Trade Winds in the northern hemisphere, and South-East Trade Windows in the southern hemisphere) which create the Hadley cell. The temperate zones (to about 60°) form the prevailing westerlies which create the Ferrel cell. The polar regions form the polar easterlies which create the Polar cell. These winds are modified by changing seasons and differences in underlying geography.
Geology
Geology is the study of the solid parts of the Earth (land). A large landmass is called a continent. A very small piece of land which is surrounded by water is called an island. An endemic species is unique to a particular area of geography.
The Earth has a radius of ~6350km. From the center of the Earth to about ~5100km from the surface (in thickness of ~1220km) is Earth’s inner core which is believed to be made mostly of solid Iron and Nickel. From the outside of the inner core to about 2890km from the surface (in thickness of ~2400km) is Earth’s outer core which is made mostly of liquid Iron and Nickel. From the outside of the outer core to ~100km from the surface (in thickness of ~2900km) is Earth’s mantle which is made mostly of hot rock. Rock is a solid mass made of minerals or mineraloids. From the outside of the mantle to the surface (in thickness of ~0-100km) is Earth’s crust. Earth’s crust is made either of oceanic crust, which is ~5-10km thick, or continental crust, which is ~30-50km thick. Oceanic crust is denser than continental crust which is why continental crust is higher than oceanic crust.
The crust and the top ~0-100km of the mantle are called the lithosphere. Underneath the lithosphere for another ~100km is the asthenosphere. The lithosphere is broken up into pieces called tectonic plates which move independently, driven by the asthenosphere.
When two tectonic plates move away from each other, the boundary is called a divergent boundary. As the plates diverge, crust and mantle from below melt into magma and extrude to form mountain ridges (which tend to be underwater), with the largest being the mid-ocean ridges (including the Mid-Atlantic Ridge and the East Pacific Rise), and the magma may also potentially form volcanic islands which breach the surface of the water. The crust is youngest at the ridge and the age increases approximately symmetrically and orthogonally to both sides of the ridge. Divergent boundaries experience shallow earthquakes which are sudden releases of energy that shake the Earth. As magma cools, certain magnetic rock (magnetite) solidifies with a particular orientation towards the magnetic pole. Symmetric, repeating stripes of rocks oriented in flip-flopping directions around a divergent boundary match the pattern of Earth’s periodic geomagnetic reversals.
When two tectonic plates move toward each other, the boundary is called a convergent boundary. As the plates converge, the denser plate performs subduction to go beneath the less dense plate which also creates a trench between them and the elevation of the less dense plate tends to increase. As the subducted plate reaches the asthenosphere, it melts. This colder mantle is denser and sinks to the bottom of the mantle. As lower material heats up, it becomes less dense and starts to rise, and these forces together create mantle convection cells. Convergent boundaries experience shallow or deep earthquakes. As the subducted plate melts underneath the less dense plate, magma plumes at the point of the melting may make their way up through the less dense plate to create islands or continental volcanoes. If a convergent boundary occurs with two plates of similar density (e.g. two continental plates), instead of subducting, the two plates may bulge vertically to create a mountain range (e.g. the Himalayas), although some crust may still subduct and create volcanoes.
When two tectonic plates largely move parallel or anti-parallel, the boundary is called a transform boundary. Earthquake activity is high and volcano formation is variable depending on various factors.
Hotspots may form volcanic regions independent of tectonic plate boundaries. If they stay fixed within the mantle, they may create island chains as the plates move above them (e.g. Hawaii).
Transform faults occur at transform boundaries or at divergent boundaries such as the mid-ocean ridges where a ridge or part of a plate has a sudden offset shift (causing an earthquake). Fracture zones are the area where the shift occurred and do not have earthquakes because they’re just the byproduct of past transform faults.
An oceanic basin is created by diverging plates and recycled by converging plates:
- The embryonic phase is an uplift from a previous convergence (e.g. East African Rift Valleys).
- As the plates diverge, the juvenile phase is when water rushes into the diverging rift valley to create a sea (e.g. Red Sea).
- The mature phase is a fully formed ocean in place of the sea along with continental margins (e.g. Atlantic Ocean).
- The declining phase is when plates converge which forms island arcs and trenches (e.g. Pacific Ocean).
- The terminal phase is when the ocean turns into sea(s) and consists of narrow, irregular seas with young mountains (e.g. Mediterranean Sea).
- The suturing phase is convergence and uplift of young to mature mountain belts and the whole process repeats (e.g. Himalaya mountains).
An oceanic basin is made of a continental shelf where the continental plate meets the ocean. The coast is the area where land meets the ocean. The shelf is underwater, shallow, and generally flat. The shelf leads to the continental slope which is a steep drop off. The continental slope leads to a less steep slope called the continental rise which contains run off that dropped down the slope (sometimes carving submarine canyons in the slope created by turbidity currents). The continental rise leads to the abyssal plain which is generally flat and makes up the the majority of the ocean basin with a depth between 3000-6000m. The continental margin consists of the continental shelf, continental slope, and continental rise.
At the coast, during the day, there tends to be a wind from the ocean onto land because land warms faster from the sun than water which creates a density gradient. At night, this tends to be reversed because water has more latent heat capacity.
A seamount is a mountain below the ocean surface of at least 1000m with a generally rounded top. Tablemounts (or guyots) are seamounts with flat tops, generally from wave erosion during a seamount’s island phase.
A fringing coral reef may form around an island. As the island is eroded, a barrier reef may remain as the island sinks. When the island submerges, an atoll with a lagoon may remain.
Hydrothermal vents, often found at mid-ocean ridges, are pores in the crust near underwater volcanoes which spew hot water and various chemicals: clear warm water vents (<30°C), white water vents (30°C - 350°C), and black smoker vents with sulfides (>350°C). Hydrothermal vents may support Hydrogen sulfide-oxidizing bacteria that perform chemosynthesis instead of using photosynthesis.
Oceanography
Oceanography is the study of the oceans on the Earth. An Ocean is a large body of saline water. Oceans formed on Earth about 4.4 billion years ago. Salinity defines the amount of dissolved inorganic solids (usually salts), and usually includes some gases (e.g. CO2) and inorganic ions, in water, as a ratio per thousand grams (‰). Typical salinity of an ocean is 35‰ or 3.5%, made mostly of Chlorine and then Sodium and then other salts, and the proportions of salts are relatively constant throughout the world (Marcet’s Principle, Forchhammer’s Principle or the Principle of Constant Proportions) even though salinity generally varies between about 33‰ and 37‰ depending on the amount of freshwater and other conditions. Brackish water has some salinity but less than 33‰. Ions are generally added to oceans by river discharge, volcanic eruptions, and hydorthermal activity. Ions are generally removed from the ocean by adsorption, sea spray, biological processes, and hydrothermal activity. Salinity approaches ~35‰ as depth increases and the depth range with a sharp change in salinity is called the halocline.
The thermocline is the depth range with a sharp change in temperature (generally where sunlight no longer penetrates). The pycnocline is the depth range with a sharp change in density produced by the effects of the thermocline and halocline. All clines occur between ~300 - ~1000m. Where there is a low or no pycnocline (e.g. polar oceans), there is significant vertical mixing of water.
Aspects of salinity:
- Temperature and salinity (and pressure) are the largest determinants of density (with temperature being the largest) and thus drive vertical water flow patterns. Salinity has the largest impact on density in polar oceans because the temperature change is so small. The average density of seawater is ~1.025 gm/cm3.
- In general, all else equal, as salinity increases, density increases. Similarly, as temperature increases, density decreases.
- Salinity generally records the physical processes affecting a mass of water when it was last at the surface, so at lower depths, it may be used as a tracer to determine its general source.
- Salinity near the surface is largely regulated by the balance between precipitation (lowering salinity) and evaporation (increasing salinity). The highest salinities occur in the hot, dry, sub-tropical central gyres (~20°-30° N/S) where atmospheric cirulation cells descend and have high evaporation and low precipitation.
- Low salinities at the equator because of rain due to rising atmospheric cirulation.
- Salinity may be affected by ice formation and melting.
- Salinity is generally lowest at higher latitudes and highest at lower latitudes, although there’s a dip at the equator because of the excess of precipitation over evaporation.
- The higher the salinity, the higher the density. When water warms, it expands and becomes less dense.
- The refractive index of water increases as salinity increases because the density increases.
- Because of the greater local heating effects of the Gulf Stream, the North Atlantic is warmer than the North Pacific, so water evaporates more rapidly. This leads to increased salinity and thus increased density and thus more down-welling of water.
A Temperature-salinity diagram (or T-S diagram) plots a water column’s temperature and salinity as observed together at specified depths (generally excluding surface waters).
Oceans are layered with different densities of water with the top layer being the Photic Zone (~0-600m) where photosynthesis may occur and the rest is the Aphotic Zone where no light penetrates. The ocean generally has a blue-ish color because blue colors are absorbed the least and travel the furthest down into the ocean or reflect up.
Ocean currents are masses of water below the pycnocline that move from one place to another, redistributing heat from warmer regions to cooler regions, either by density-driven circulation or wind-driven cirulation. Density-driven circulation occurs because cold and dense deep waters (e.g. polar oceans) sink to the bottom and move horizontally into less dense, warmer areas. Wind in the air above oceans is primarily formed by heating from the sun. Ocean currents are carried 90° to the right of the wind in the northern hemisphere and 90° to the left of the win in the souther hemisphere due to Ekman transport. These currents in the context of the winds create large circular gyres in the major oceans. These are also major drivers of current flows such as the Gulf Stream. Eddies are small areas of an ocean that have a different temperature than surrounding water.
Upwelling is the vertical movement of cold, deep, nutrient-rich water to the surface. Downwelling is the vertical movement of surface water to deeper parts of an ocean which carries disolved Oxygen to the sea floor. Upwelling water to a coast brings nutrients (e.g. those that fell all the way to the sea floor) and gases (which tend to pack down in colder, denser waters; e.g. O2, CO2, etc.) towards the surface. Due to the transport of nutrients, areas of high upwelling are areas with high productivity (e.g. coastal areas with ekman flow away from the coast, at the equator, offshore winds, sea floor osbtructions, and sharp bends in coasts) and areas with high downwelling are areas with low productivity (e.g. coastal areas with ekman flows towards the coast and the middle of gyres due to the inward and downward pressure).
There are five major oceans: the Pacific Ocean, the Atlantic Ocean, the Indian Ocean, the Southern Ocean, and the Arctic Ocean. In general, the equatorial Pacific follows the gyre’s path of warm water on the west going up to the arctic and cold water coming down the east. During an El Niño, high pressure along the South American coast causes trade winds to diminish or reverse and with this change, a large area of warm water moves from the west towards the east which significantly changes upwell and downwelling and nutrient availability. A La Niña is the opposite where more cold water migrates West.
pCO2 is the partial pressure of CO2, so as pCO2 goes up, the concentration of CO2 goes up, and vice versa. As the concentration of CO2 goes up, there is an increase in reactivity between H2O and CO2 which increases the concentration of Carbonic Acid (H2CO3), some of which then reacts to form Bicarbonate (HCO3-) and Hydrogen ions (H+), and some of the Bicarbonate reacts to form Carbonate (CO32-) and Hydrogen ions (H+). These additional H+ ions thus decrease pH (because it is a negative logarithmic scale of H+ ion concentration). However, up to a point, Carbonic Acid is a weak acid and may buffer CO2 which generally keeps the ocean alkaline.
In the northern hemisphere, air around a high pressure area moves clockwise, and air around a low pressure area moves counterclockwise. A hurricane (or cyclone or typhoon) is a large, low-pressure area in the tropics moving west with the trade winds with strong winds and rains which devlop by picking up energy from warm water and surface winds feed moisture into the storm. The power of a hurricane comes from the latent heat of condensation as water vapor condenses into rain.
The Benthic Zone is the area where water reaches underlying land, and organisms in this zone are called Benthos which live on, in, or attached to the sea floor and eat dead organic matter called Detritus falling down from above, or they are suspension feeders, or they eat other organisms in the benthos.
The Hadal Zone is the area of ocean below 6km in deep ocean trenches.
The Pelagic Zone is the area of water above the benthic and hadal zones.
Tides are daily raising (high tide) and lowering (low tide) of ocean levels due to gravitational pull of the Moon (primarily) and Sun and other planets and the rotation of the Earth. There may be one or two each high and low tide per day depending on various factors and positions of the Moon and Sun.
Plankton are organisms that move due to oceanic currents and are too small to counteract such currents. Phytoplankton are autotrophic. Zooplankton are heterotrophic.
Biosphere
The Biosphere consists of all life on a planet and is made of ecosystems. An Ecosystem consists of all life and their Environment (the things life interacts with in a particular area).
An area of vegetation has many layers and the topmost layer is called the Canopy.
One or more ecosystems may be classified as part of Biomes based on terrestrial vegetation type or aquatic physical characteristics (with intersections called Ecotones):
- A Tropical Rainforest biome has relatively constant and high rainfall throughout the year.
- A Savanna biome has relatively warm and constant temperature throughout the year with some seasonal variation.
- A Northern Coniferous Forest (or Taiga) biome has a seasonal climate and consists largely of Conifers (gymnosperms, cone-bearing seed plants).
- A Temperate Broadleaf Forest biome has a seasonal climate and consists largely of deciduous trees which drop their leaves before winter.
- A Temperate Grassland biome has a seasonal climate and consists largely of grass.
- A Desert biome is an area of land with little precipitation.
- A Chaparral biome has a seasonal climate and consists largely of shrubs and small trees.
- A Tundra biome has a seasonal but cold climate.
Aquatic biomes are largely characterized by chemical or physical properties:
- A Lake biome is a large body of water other than an ocean. Oligotrophic Lakes are Oxygen-rich and Eutrophic Lakes are Oxygen-poor depending on depth, season and ice cover. Some plants may be rooted in shallow, well-lit areas near the shore of a lake called the Littoral Zone. Away from shore but still bathed with light is the Limnetic Zone.
- A Wetland biome is an area that is submerged in shallow water at least part of the year.
- A River or Stream biome is characterized by a high speed flow of water.
- An Estuary biome is a transition area between a river and ocean.
- An Intertidal Zone biome is a shore area that is periodically submerged and exposed by the tides.
- An Oceanic Pelagic Zone biome is a large area of an ocean away from shore that is constantly mixed by wind driven currents.
- A Coral Reef biome is a shallow marine area with Calcium Carbonate skeletons formed by Corals.
- A Marine Benthic Zone (or Neritic Zone) biome consists of a seafloor area away from shore but still well-illuminated.
History of Life
Fossils are remains or traces of dead organisms. Paleontology is the study of fossils. In some cases, dead organisms settle into layers of sediment which are compressed over time on top of each other, with layers called strata (singular stratum).
The geological record is an analysis of strata and fossils to date different periods of the Earth’s history:
- Hadean (~4.6 billion years ago)
- Archaean (~4 to ~2.5 billion years ago): The first prokaryotes existed at least 3.5 billion years ago.
- Proterozoic (~2.5 billion years ago to ~540 million years ago): The first eukaryotes existed at least 1.8 billion years ago. The first land-based eukaryotes existed at least 1.3 billion years ago. The first multi-cellular eukaryotes existed at least 1.2 billion years ago. The split of plants and animals occurred at least 1 billion years ago.
- Paleozoic
- Cambrian (~540 to ~485 million years ago): The first signs of predation. Larger forms of life such as fungi, plants and animals colonize land ~500 million years ago.
- Odrovician (~485 to ~444 million years ago)
- Silurian (~444 to ~419 million years ago)
- Devonian (~419 to ~359 million years ago)
- Carboniferous (~359 to ~299 million years ago)
- Permian (~299 to ~252 million years ago): At the end of the Permian, the Earth had a single continent called Pangaea.
- Mesozoic
- Triassic (~252 to ~201 million years ago)
- Jurassic (~201 to ~145 million years ago): About 175 million years ago, Pangaea began to break apart into the northern continent of Laurasia and the souther continent of Gondwana. First mammals ~180 million years ago.
- Cretaceous (~145 to ~66 million years ago)
- Cenozoic
- Paleogene
- Paleocene (~66 to ~56 million years ago)
- Eocene (~56 to ~34 million years ago)
- Oligocene (~34 to ~23 million years ago): The first primates.
- Neogene
- Miocene (~23 to ~5.3 million years ago): Earliest direct human ancestors. About 20 million years ago, the modern 7 continents were approximately where they are now.
- Pliocene (~5.3 to ~2.6 million years ago): The first bipedal human ancestors.
- Quaternary
- Pleistocene (~2.6 million years ago to ~11 thousand years ago): The first Homo Erectus.
- Holocene (~11 thousand years ago to present)
- Paleogene
Noteworthy
Seabirds (or Marine Birds) have Supraorbital Glands (or Salt Glands) near the nose which secrete excess salt from consumed seawater.
Ferns are vascular but seedless plants with an independent gametophyte generation (meaning the gametophyte can live independently of the parent sporophyte, unlike seed-based vascalar plants that have a dependent gametophyte generation).
Ecology
Ecology is the study of interactions between organisms and the environment.
Demography is the study of patterns of population density, disperson, birth, death, immigration rates, and emigration rates of a population over time.
Ecological Succession is the process of large changes in an ecology after a large disturbance or the creation of a new habitat. Initial organisms (as part of Primary Succession) are usually prokaryotes and protists, followed by photosynthesizers and the soil slowly becomes more rich from the detritus of the early successors.
A Keystone Species is a species that has a disproportionately large effect on its environment relative to its abundance.
Interspecific Competition is competition among different species in a particular area for the same limited resources.
A species has a Cosmopolitan Distribution if its range extends through most parts of the world which are habitable.
The change in a population (ΔN) over a period of time (Δt) equals the number of births (B) minus the number of deaths (D): ΔN/Δt = B-D; or, representing the difference between births and deaths as R: ΔN/Δt = R. If there are no density-dependent forces at play (idealized), then the per capita change in population size for the given time period (rΔt) is the contribution that the average member of the population makes to the increase or decrease of the total population and R may be expressed in terms of the total population changing based on this rate: R = rΔt × N and thus for some time interval: ΔN/Δt = rΔt × N. This may be expressed in calculus terms as dN/dt = r × N where r is the intrinsic rate of increase.
Punctuated Equilibrium occurs when there are bursts of speciation with relatively unchanging species in between.
The Island Equilibrium Model (or the MacArthur-Wilson Model, or Insular Biogeography) observes that the number of species in an isolated ecosystem stabilizes at a dynamic equilibrium related to rates of immigration and extinction/emigration, the migration distance to the ecosystem from other ecosystems, and the size of the ecosystem.
Laboratory Techniques
General Techniques
An assay is a scientific analysis of some substances.
In vitro describes an experiment run in or on glass and outside an organism’s normal environment, whereas in vivo describes an experiment run on an organism in its normal environment.
A Bunsen burner creates a flame of about 1,000 °C which is used for sterilization and heating. Expanding air around the bunsen burner also drives some microorganisms in the air away from the work area, which is about 1-2 feet in radius around the burner. An inoculation loop may be used to transfer microorganisms in a liquid: first, sterilizing the loop in the bunsen burner, letting the loop cool near the burner, then using the loop for transfer, and then sterilizing the loop again.
Double stranded DNA may be split into two, single-stranded DNA molecules often by increasing temperature. The process of complementary strands of DNA or RNA combining together under proper conditions (e.g. temperature) is called nucleic acid hybridization or annealing.
A DNA Library is a collection of DNA framents stored in a population of organisms (e.g. DNA plasmids in yeasts). A cDNA Library is a DNA library of mRNA (without introns) which has been converted into Complementary DNA (cDNA) using reverse transcriptase.
Replica Plating is a technique that transfers cells from one plate to another in the same spot as the last plate with different growth media on each plate and the plates can be compared to each other to determine which growth media cells can and cannot grow on.
X-Ray Crystallography focuses an X-ray beam through a substance on a detector that creates a 3D structures from the defraction of the X-ray beams by the atoms of the substance.
Aliquot
An aliquot is a sample of a larger whole, usually an exact divisor. For example, a 10mL sample of a 100mL solution is an aliquot.
Pipette
A pipette (or pipet) is used to transfer a measured volume of liquid. Disposable tips are used to reduce cross-contamination. Common pipette ranges are: P10 = 0.5μL - 10μL, P20 = 2μL - 20μL, P200 = 20μL - 200μL, P1000 = 200μL - 1000μL, and P5000 = 1000μL - 5000μL.
Procedure:
- If necessary, set the dial of the pipette to the desired volume.
- Add a disposable tip.
- Press down to the first “stop”.
- Put the pipette in the source solution.
- Release the pipette stopper to take up the configured amount of solution.
- Put the pipette in the target location and press down past the first stop and all the way to the second stop.
Dilution
Serial Dilution
A serial dilution starts with a solution and iteratively dilutes the solution by a constant factor from the previous dilution to create a logarithmically decreasing concentration of the original solution. For example, if the starting solution is some amount of something per XmL (e.g. 500,000 cells / mL in 100mL), and the dilution factor is 10, then the second solution should be 50,000 cells / mL in 100mL, the third solution should be 5,000 cells / mL in 100mL, and so on. To achieve this, each subsequent solution starts with (X - X/10) base solution (e.g. water, media, etc.) with nothing in it. For example, the second solution starts with (100mL - 10mL) = 90mL of base solution. Then, X/10 of the first solution is withdrawn and placed into the second solution. For example, 10mL of the first solution is taken and put into the second solution. Before every transfer, the source solution should be mixed (e.g. by pipetting up and down). Next, 10mL of the second solution is put into the third solution, and so on.
Ratio Dilution
If you start with a solution with a concentration of some substance C1 and you want to get to concentration C2 in volume V2, then use the formula C1 × V1 = C2 × V2 and solve for V1 to figure out how much to take out of the original solution and put into V2.
If the resulting volume would be too small to accurately extract (e.g. &lgt; 1μL), then perform a serial dilution with a maximum dilution of 1,000 fold at every step:
- Find the ratio of concentrations using
C1 / C2. - Iteratively divide this ratio by 1,000 to create the dilution steps. For example, if the ratio is 40,000, then the first dilution is by 40 fold and the next dilution is by 1,000 fold.
- For each dilution step, remove X amount of the previous solution and put into a solution of
Y - Xsolution (e.g. PBS, etc.) whereX + Y = ratio. For example, if a dilution step is to dilute by 40 fold and the starting concentration is100 μg/mL, then take 1μL (X) of this solution and add to 39μL (ratio-X) to create 40μL of100 / 40 = 2.5μg/mLsolution. - At any point, use the
C1 × V1 = C2 × V2formula. In the above example, to perform the last 1,000 fold dilution, C1 = 2.5μg/mL, C2 = 2.5ng/mL (divide by 1,000), and V2 = 10mL (for example), then V1 = 10μL.
Standard Curve
A standard curve plots multiple points of known quantities (e.g. a logarithmic concentration on the x-axis and some value on the y-axis) and then may be used to estimate a y-value based on some x-value by curve-fitting and extrapolation.
Microscopes
Magnification is the process of increasing the apparent size of something such as by focusing through the use of optics by using a lens to bend light rays and focus them on a focal point thus enlarging an image.
A light microscope focuses light through a substance which refracts through a glass lense so that it’s magnified. Light microscopes cannot resolve substances less than ~200nm.
A compound light microscope uses multiple lenses to sequentially magnify an image. The microscope eyepieces, the ocular lenses, typically magnify 4X - 10X. Another lense called the objective lens typically magnifies 4X - 100X. The total magnification is the product of these two magnifications. Typically, compound light microscopes have a magnification up to 1,000X.
A light microscope’s iris diaphragm may be used to create contrast between an object and its background by controlling the amount of light passing through. The condenser focuses light onto the stage. The aperture controls how much light is emitted.
A parfocal lens stays in focus when manification or focal length are changed. When changing objective lenses, use the grip on the carousel instead of pushing the lenses themselves.
A confocal microscope uses point illumination to detect a small portion of a sample and this is repeated at different depths and points to create a 2D or 3D picture.
A Bright-field microscope uses illumination from below and observation from above with a white light and contrast is created by attenuating light in dense areas.
An inverted microscope has the light source and condensor on the top and lenses at the bottom. An inverted phase contrast microscope is often used to view cells with the phase contrast necessary to accentuate differences in densities since cells are mostly water and clear.
A fluorescence microscope detects the fluorescence of fluorophores (or reporter molecules, or probes) in a material.
An electron microscope (EM) focuses a beam of electrons through a substance onto a surface and use a magnetic field to have a similar effect as the bending of light in a light microscope. Typically, electron microscopes have a magnification up to 1000,000X. Electron microscopes cannot resolve substances less than ~2nm.
A scanning electron microscope (SEM) focuses a beam of electrons on a substance and detects the interaction of atoms of the substance with the electron beam to create a 3D view.
A transmission electron microscope (TEM) focuses a beam of electrons through a thin substance and onto a surface to understand the internal structure of a substance.
The lenses of a microscope should be cleaned by a sterile cotton swab dipped in isopropanol and then dried with lens paper.
When looking through a microscope with two lenses, use both eyes, open them wide, try to move eyelashes away, and then adjust the distance between the two oculars so that both lenses are in view at the same time. When first looking at a slide, drop the stage as far down as possible using the coarse adjustment knob, start with the lowest objective lens (typically, 4X for eukaryotes, and 10X for bacteria), increase the aperture to turn the light intensity down, then bring the stage up using the coarse adjustment knob until the object comes into focus, and then use the fine adjustment knob to finish focusing. If it is a parfocal microscope, next, switch objective lenses to increase magnification and only use the fine adjustment knob to fine-tune the focus. When increasing magnification, consider reducing the aperture to increase light intensity. Before switching to the 100X objective lens, move the 40X lens away mid-way to the 100X lens and then use a dropper to add a drop of immersion oil onto the slide which eliminates the air gap between the slide and lens that could scatter light, pass the 100X lens through the oil past the lock point and back to help spread the oil, and then adjust the fine adjustment knob. To finish, use the coarse adjustment knob to bring the stage down and clean the oil off the lenses.
One of the microscope lenses will have a built-in ocular micrometer that shows a ruled scale with 100 divisions. These divisions have a different length depending on total magnification: 40X = 25 microns, 100X = 10 microns, 400X = 2.5 microns, 1000X = 1 micron.
A microscope slide is a thin piece of glass used with microscopes and a slide is typically 75mm long x 26mm high x 1 mm thick.
Phosphate-buffered saline
Phosphate-buffered saline (PBS) is an isotonic, water-based salt solution with osmolarity and ion concentrations similar to those in a human. Therefore, PBS is non-toxic to most human cells and may be used to wash them.
Dimethyl sulfoxide
Dimethyl sulfoxide (DMSO) is frequently used as a solvent in biology.
Biosafety Cabinet
A biosafety cabinet (or biohazard hood) is a mostly enclosed workspace for working with sensitive biological materials. Air is circulated through the cabinet and exhaust air is filtered to remove biological material. At the area where the human puts their hands through, there is air flow to reduce particals from getting in or getting out. Latex gloves should be used inside the hood and they should be spritzed with 70% ethanol to clean them before putting them inside. Some hoods have an Ultraviolet light function to sterilize all materials of biological agents after use.
Plating
A petri dish (or plate) is a glass or plastic covered plate used to grow (or culture) microbes.
Common experimental cells include:
- Fibroblast cells which create the extracellular matrix and are migratory, adhesive, and relatively easy to grow because they must naturally be able to live, divide, and function in a multitude of environments in a body.
Petri dishes may be coated with a solid, liquid, or semi-solid growth medium (or culture medium) with nutrients that microbes may use to grow.
Common media include:
- Agar plates with a high melting point of 85°C (agar is a jelly from red algae made of 70% agarose [a polymer of galactose] and 30% agaropectin [various small molecules such as sulfates and pyruvates]).
- Minimal Essential Medium (MEM) and variants such as DMEM which has glucose, salts, amino acids, and vitamins.
- DMEM + 5-20% animal serum such as fetal bovine serum (FBS) + antibiotic + antimycotic: The fetal serum includes growth factors and cytokines to help signal cells to grow (as if exposed to natural blood serum). The antibiotic and antimycotic reduce the chances of bacteria or mold to grow in the medium.
Use a marker to write on the outside of the non-cover part (in case the cover falls off) before use with a date, time, and description. When not in use, tape the lid to the plate and write the same information on the tape. Plates are stored “upside down” (agar up; lid down) to avoid condensation (e.g. from temperature changes or microbe metabolism) falling back down on the plate.
Cell Feeding
Depending on the cell type, cells may need to be fed, for example, every few days with new media.
In general, mammalian cells grow (or incubate) best in a 37°C environment with 5% CO2 concentration. Normal air CO2 concentration is about 0.05%; however, in the microenvironment of an organism, CO2 concentration is about 5%.
Culture Splitting
Culture splitting (or subculturing or passaging) is used to reduce the cell concentration (or confluency) in a culture and replace some of the media. This is required to reduce concentrations of toxic materials and dead cells, give space for cells to grow, reduce the chances of mutations due to space pressures, etc. The passage number is the number of times that the remnants of a culture have been split. In general, cultures should be split before confluency reaches 80%.
Cell adherence
Some cells adhere to the surface of the plate. To de-adhere cells, a solution is used of 0.25% trypsin with Ethylenediaminetetraacetic acid (EDTA) in a balanced salt solution without Ca and Mg. EDTA chelates divalent cations such as Ca++ and Mg++ which are used as part of adherence. Trypsin acts as a membrane-impermeable enzyme that cleaves the cell membrane proteins that participate in adherence.
This solution is stored in a refrigerator and thawed for about 10 minutes before use. After removing media from a solution and before applying trypsin, wash cells with PBS to remove material that may inhibit trypsin. After adding trypsin, incubate in 37°C / 5% CO2 for about 5 minutes to complete the de-adherence.
Hemocytometer
A hemocytometer is used to estimate the number of cells in a fluid (originally created for blood cells and thus the heme- prefix). It is a glass plate with a chamber designed for a specific amount of fluid and thus a fixed volume. The glass normally has laser-etched squares of dimensions of, for example, 1mm wide x 1mm tall x 0.1mm depth, or 0.1 mm3, or 10-4 mL. Estimate the number of cells per mL:
- Count the number of cells per square in a few of the squares.
- Take the average of these counts.
- Multiply the average by 104 to calculate the approximate number of cells per mL.
Fixation
Fixation is the process of preserving biological cells and tissues by introducing a substance that minimizes biological reactions. It is generally done in preparation for staining (discussed in the next section). Fixation may be done by washing with PBS and then adding 95% ethanol solution for about 10 minutes.
Mounting media may be used on a microscope slide to solidify cells’ positions. Examples include Permount, glutaraldehyde fixative solution, aqueous mounting medium (Anti-Fade), etc.
Heat-fixing is used to dessicate and kill bacteria to preserve their shape and size, and firmly attach them to the slide to withstand the wash steps during staining.
Formalin-fixed paraffin and paraformaldehyde are often used to fix antigens.
Staining
Acidic dyes (e.g. nigrosin) have a negative charge which repels the negative surface charge of bacteria. Such dyes may be used as a negative stain where the background is stained and the organisms are not.
Basic dyes are positively charged and attracted to most bacteria. A simple stain uses a single dye (e.g. methylene blue) to visualize size, shape, arrangement, and structure of any organisms on a plate.
A differential stain uses multiple dyes to identify different classes of organisms. An acid-fast stain identifies bacteria with a waxy outer coating (e.g. Mycobacterium, Nocardia).
A structural stain helps visualize bacterial structures such as flagella, endospores, and capsules.
DAPI is a fluorescent dye (presenting as blue) that binds to Adenine and Thymine rich regions such as DNA. It can pass through cell membranes and the nuclear membrane.
A Coplin jar has grooves to hold microscope slides.
Hematotoxylin and Eosin staining
Hematotoxylin and Eosin (H&E) staining is used to visualize tissues (or adherent, spread out cells) with bright-field microscopes and often used on biopsies. Hematoxylin binds to nucleic acids and stains purple. Eosin (combined with Orange G) stains cytoplasmic material in a lighter purple. This stain helps to visualize the concentration of cells.
A Papanicolaou stain (pap stain) is an H&E stain used as part of a pap smear to help detect cancerous cells. The stain is evaluated by comparing the concentration and/or structure of cells as compared to non-cancerous baselines.
Lipid Fluorescent Dye Labeling
Lipid fluorescent dye labeling is a staining procedure which uses lipophillic, fluorescent dyes that preferentially intercalate into different organelle membranes. Carbocyanine is a fluorophore that preferentially intercalates in membranes such as the Endoplasmic Reticulum.
Immunohistochemistry
Immunohistochemistry (IHC, or immunofluorescence) uses an antibody that targets something in the cell and couples the antibody to a fluorophore (together called a fluorescent antibody) or another chemical (e.g. HRP) that creates a colored dye when stained. Example fluorophores are Alexa488 (emits green when excited by blue), Alexa568 (emits red when excited by yellow/green), fluorescein (emits red when excited by yellow/green), rhodamine (emits red when excited by yellow/green), FITC, and Texas red.
Indirect immunohistochemistry uses a “primary antibody” or anti-A (with anti- standing for antibody and A being the antigen or target cellular structure) along with a “secondary antibody” that binds to the primary antibody. The secondary antibody has the fluorophore or dye-related substance attached. This technique is popular because the process of creating the secondary antibody is not simple and yet it has a generic target of the primary antibody rather than the specific antigen targeted with the primary antibody which may be separately made. This may reduce costs and the targeting of a more pronounced primary antibody may amplify the microscopy signal.
Direct immunohistochemistry uses a single antibody with fluorophore or dye-related substance attached. It lacks some of the benefits of indirect immunohistochemistry and generally shows a dimmer image, although it tends to be a simpler procedure.
Immunocytochemistry (ICC) is like immunohistochemistry but focused on just the cells without anything else such as extracellular matrix.
Bovine Serum Albumin (BSA), Normal Goat Serum (NGS) and others are proteins used as a blocking buffer which bind non-specific binding sites and increases the chances that antibodies will only bind to antigens of interest.
A positive control is used to test a protocol without introducing an experimental variable. A negative control is used to test the specificity of an antibody.
Viability assay
A viability assay studies cells and tissues under experimental conditions. Examples include observing proliferation and death depending on growth factors (proliferation assay), toxicity (cytotoxicity assay), drug effectiveness, etc. These assays generally work by introducing a reagent that interacts with metabolic chemicals produced by living cells such as ATP, NADH, etc. The reacted product has fluro- or chemiluminescence that may be observed by microscopy. The amount of color change is an approximation of the amount of cells and their liveliness.
One common viability assay is the MTS assay (or WST-8) in which MTS tetrazolium is reduced by NADH-dependent dehydrogenase enzymes in metabolically active cells (NADH production) and generates a formazan dye which can be measured in the 490-500nm range (orange). A similar assay is the MTT assay which has additional lysis and washing steps.
Electrophoresis
Gel electrophoresis determines the size of DNA, RNA, or proteins by moving them through a gel and buffer using electricity. A solid but porous gel (e.g. Agarose or Polyacrylamide) is hardened in a container with a comb that is removed after solidification and leaves small wells in a row where the target substances will begin. The gel is submerged in a liquid buffer (e.g. TAE or TBE) which can carry electrical current. A negative electrode (cathode) is placed on one side of the container and a positive electrode (anode) on the other side. Target substances are injected into the wells, each defining a lane. A reference substance called a ladder is placed in the first lane which will present known sizes as reference for the other lanes. When an electrical current is turned on for a period of time, target substances will travel towards their opposite charge through the gel (e.g. negatively charged DNA and RNA travel toward the anode). Target substances are dyed for visualization with a UV light. The location of the substances in each lane, and relative to the ladder, allow for estimation of the length of the substances (e.g. number of base pairs for DNA). Larger molecules move more slowly through the gel than smaller molecules. Double stranded DNA tends to be linear and simple to analyze whereas single-stranded nucleic acids or proteins need to be denatured with substances such as NaOH for single stranded RNA or DNA, and SDS (or SLS) for proteins (which also gives a predictable negative charge).
An electrophoretic mobility shift assay determines if certain proteins can bind to certain DNA or RNA molecules. This uses gel electrophoresis with a control sample without proteins as the ladder, and in the other lanes, if proteins bind to the nucleic acids, then the substance will be large and move more slowly through the gel.
Blots
A Southern blot is used to detect if a mixture of DNA contains a specific DNA sequence. Restriction enzymes are used to break the DNA into pieces. The DNA mixture is separated by size using gel electrophoresis. The gel is combined with a sheet of positively charged Nylon or Nitrocellulose using pressure and baked. A hybridization probe is a complementary DNA strand that is used to search for the specific sequence. It is made fluorescent or radioactive for detection. The hybridization probe is applied to the gel and Nitrocellulose plate and time is given for any potential annealing. The plate is then washed and if the probe is detected, then annealing occurred and the sequence was present in the original sample.
A Northern blot is similar to a Southern blot, but it’s for RNA.
A Western blot is similar to a Southern or Northern blot, but it’s for proteins, and antibodies are used as the hybridization probe.
Polymerase Chain Reaction
Polymerase chain reaction (PCR) exponentially replicates DNA sequences using heat cycles. Each product (or source) of a replication is called an amplicon. Synthesized DNA primers target the beginning and end sequences of the desired DNA for replication. Heat is increased until double-stranded DNA is split into single strands. Heat is lowered and the primers anneal to the 3’ ends of the single strands. DNA polymerase (such as Taq) duplicates the two primed strands. Heat is increased to start the cycle again, and given enough nucleotide materials, the sequences keep doubling through each cycle.
Quantitative Real-Time PCR
Quantitative Real-Time PCR (qPCR) monitors the rate of amplication of PCR in real-time by detecting fluorescence of a fluorophore that binds to double-stranded DNA (e.g. SYBR Green). If done in combination with a housekeeping gene with a known rate of amplification, then the relative amount of DNA can be estimated.
Reverse Transcription PCR
[Reverse Transcription PCR][] (RT-PCR) uses reverse transcriptase to create cDNA from RNA which is then processed through PCR. RT-PCR may also be used with qPCR (qRT-PCR) for quantitative real-time reverse transcriptase PCR. Generally, “RT” in this context is reserved for use as an acronym only for Reverse Transcription rather than Real Time as that may cause confusion.
Enzyme-Linked Immunosorbent Assay
An Enzyme-Linked Immunosorbent Assay (ELISA) measures amounts of a ligand such as a protein (e.g. an antibody or antigen) in a liquid. First, an antibody is bound to the target ligand and also bound to the enzyme Alkaline Phosphatase. Next, the substrate P-Nitrophenyl Phosphate (pNPP) is added which bind to Alkaline Phosphatase which removes a phosphate group from pNPP which causes it to turn yellow.
Methotrexate
Methotrexate (or amethopterin, MTX, trexall, rheumatrex, otrexup) has a structure similar to folic acid and blocks enzymes that require folic acid as a co-factor. For example, folic acid is a key co-factor for enzymes involved in tetrahydrofolate synthesis which is required to synthesize the nucleoside thymidine (which includes the nucleobase thymine). Inhibiting thymidine production reduces or eliminates DNA replication. Therefore, methotrexate may be used to reduce cell division (and thus sometimes used as a chemotherapy drug).
Cryosectioning
Cryosectioning is preparation of thin cross-sections (generally ~10 microns deep) from frozen tissue samples onto slides. The sections are cut from the tissue using a microtome slicer mounted in a cryostat. The tissue is first placed within a mold and into a jell-like Optimal Cutting Temperature (OCT) compound which preserves the tissue when frozen (generally at -20°C; same as dry ice). When ready for cryosectioning, the mold and tissue are secured into the cryostat (generally at -15°C to -20°C) in a chuck.
Practical Biology
Companies/Projects
- eGenesis: Dr. George Church’s company that uses edited porcine stem lines for human xenotransplantation
- Twist Bio: Synthetic biology for applications ranging from novel materials to data storage
- Denali: Age related neurodegenerative disorders such as Parkinson’s and Alzheimer’s
- Benchling: Molecular biology and gene editing design software
- Synthego: Gene knockout kits
- The ODIN: Biohacking gear
- Calico: Research and development company to increase our understanding of the biology that controls lifespan
- Infinome: Citizen science experiment bringing together data to understand the interactions between genetics and lifestyle for the good of science
- SENS: transforming the way the world researches and treats age-related disease
- Ginkgo Bioworks: using genetic engineering to produce bacteria with industrial applications
Research
- Correction of a pathogenic gene mutation in human embryos
- How the immune system could stymie some CRISPR gene therapies
Funding
Bioinformatics
- Biostars: Bioinformatics Q&A site
Purchasing
Cancer
A neoplasm (or tumor) is an excessive and abnormal growth of a tissue that forms a large mass, often proximately due to excessive cell division or abnormal cell death. If a tumor has cells with significant genetic changes that enables tumor cells to invade and disrupt functions of one or more organs (e.g. abnormal metabolism, unusual numbers of chromosomes, etc.), then it’s called a cancer (or malignant tumor). If a cancer spreads to another part of the body, then it has metastasized. If a tumor does not spread, it’s called a benign tumor. An example of a common benign tumor is a mole. Some benign tumors may not be benign in effect and may cause damage such as benign brain tumors.
A proto-oncogene is a gene that has the potential to cause cancer through mutation or over-expression. Once a proto-oncogene leads to cancer, it becomes an oncogene.
Examples of oncogenes:
- Src (or c-Src or sarc): Encodes Tyrosine Kinase. Over-expression of the SRC gene may cause cancer by activating other signaling proteins.
- Ras (or K-ras): Encodes the Ras G-Protein which relays a growth factor signal to a protein kinase cascade which stimulates the cell cycle. Mutations in Ras may cause over-production of the Ras protein which can trigger the protein kinase cascade without growth factor.
Tumor-suppressor genes encode proteins that help prevent uncontrolled cell growth.
Examples of tumor-suppressor genes:
- p53: Synthesizes to a p53 protein which is a transcription factor that promotes the synthesis of DNA repair proteins and cell cycle-inhibiting proteins (through genes such as p21), or promotes the synthesis of apoptosis genes if DNA repair is impossible.
- APC: Regulates cell migration and adhesion.
- BRCA1 and BRCA2: Regulate DNA damage repair. Certain mutations increase susceptibility to breast cancer.
- RB: Synthesizes the retinoblastoma protein which inhibits cell cycle progression from G1 phase to S phase until a cell is ready.
- SMAD4
Some viruses may cause cancer such as the Epstein-Barr Virus, Papillomavirus, and HTLV-I.
The Ames Test applies a chemical to a Salmonella Typhimurium bacteria which is auxotrophic for Histidines to see if the chemical causes excessive mutations be creating mutants that are prototrophic.
Deep Dives
Fibroblast Cells
Fibroblast migration, proliferation, differentiation, and distribution of extracellular matrix can be stimulated by various cytokines such as:
- Transforming growth factor-beta 1 (TGF-β1): Activates the genes for fibronectin and alpha smooth muscle actin (α-SMA). The PPAR gamma agonist, Rosiglitazone, prevents TGF-β1 induced fibrosis secretion of fibronectin and α-SMA.
Fibrosis (and fibrotic scarring) is a disease in which excess fibroblast cell activity leads to excess deposition of extracellular matrix components such as collagin or fibronectin. This may impede the functioning of nearby tissues because of excess thickening around the tissue affecting growth and healing or due to excess tension in connective tissue.
Appendix
Noteworthy Human Diseases
Anatidaephobia: The fear that somewhere, somehow, a duck is watching you.
Feedback
Methodology/Meta
- Every time a “context switch” occurs into this material after some time away, re-reading the entire page before re-starting is helpful. Along with the process of writing itself, this seems to maximize retention.